Taste Masking of Dexketoprofen Trometamol Orally Disintegrating Granules by High-Shear Coating with Glyceryl Distearate
Abstract
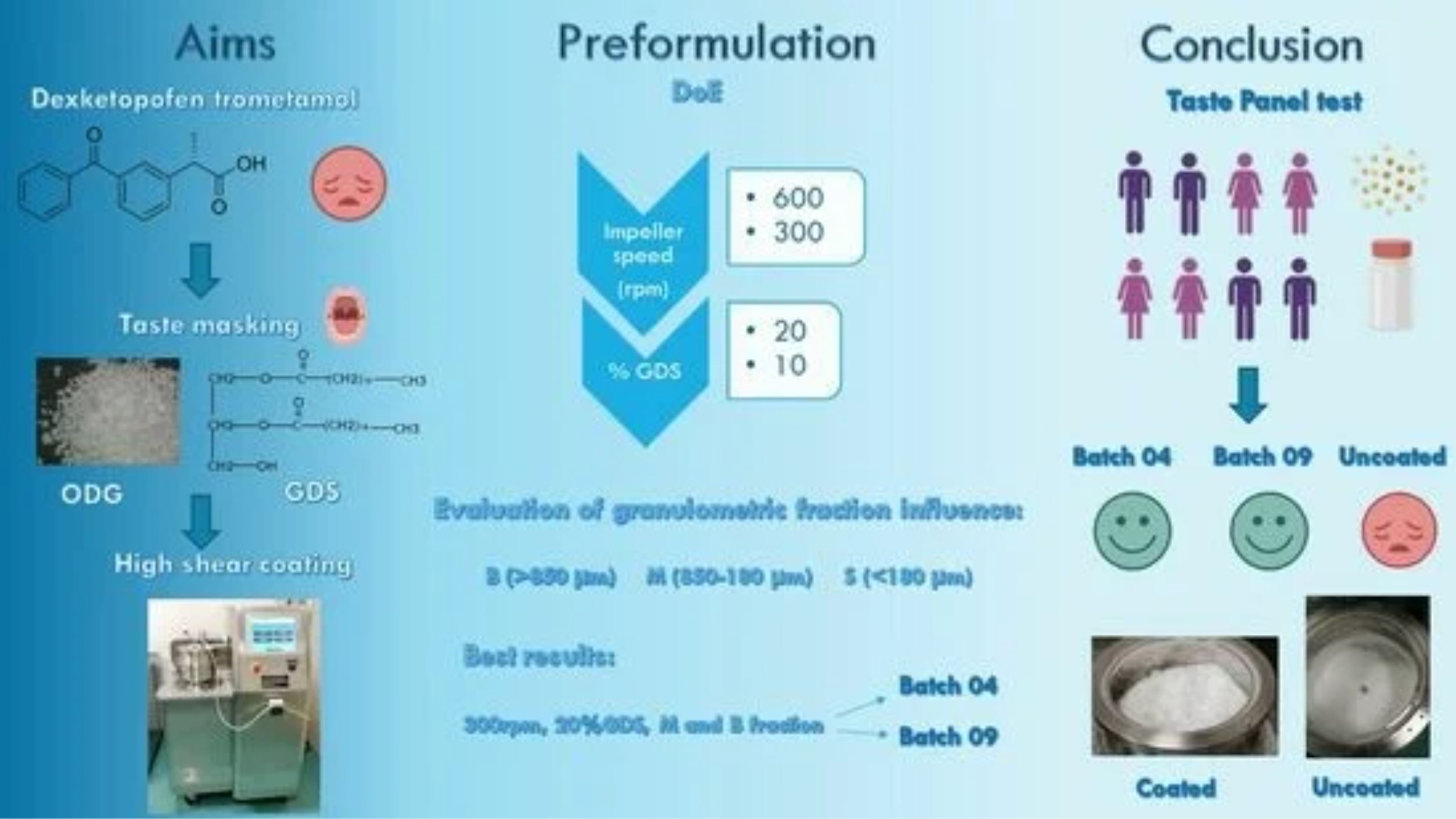
Orally disintegrating granules (ODGs) are a pharmaceutical form commonly used for the administration of NSAIDs because of their easy assumption and fast dispersion. The development of ODGs is not easy for drugs like dexketoprofen trometamol (DXKT), which have a bitter and burning taste. In this work, high-shear coating (HSC) was used as an innovative technique for DKXT taste masking. This study focused on coating DXKT granules using the HSC technique with a low-melting lipid excipient, glyceryl distearate (GDS). The HSC technique allowed for the coating to be developed through the thermal rise resulting from the friction generated by the granules movement inside the equipment, causing the coating excipient to soften. The design of the experiment was used to find the best experimental coating conditions in order to gain effective taste masking by suitably reducing the amount of drug released in the oral cavity. The influence of the granule dimensions was also investigated. Coating effectiveness was evaluated using a simulated saliva dissolution test. It was found that low impeller speed (300 rpm) and a 20% coating excipient were effective in suitably reducing the drug dissolution rate and then in taste masking. The coated granules were characterized for their morphology and solid-state properties by SEM, BET, XRPD, DSC, and NIR analyses. A human taste panel test confirmed the masking of DXKT taste in the selected batch granules.
Introduction
Patients’ compliance with oral drug assumptions is influenced by many aspects, including pharmaceutical form, daily dose, size, and ease of swallowing, with the last aspect being of particular significance for pediatric and elderly patients [1].
Orally disintegrating granules (ODGs) are multiparticulate dosage forms where the drug dose is fractionated into multiple small-sized granules and packaged into single-dose sachets named stick packs. These are becoming more and more used for their ease of assumption and fast dispersion within the oral cavity without requiring water ingestion [2]. These aspects are particularly important in the case of pain medications, allowing their prompt intake “on the go”, anywhere and at any time, and providing a fast onset of action [3]. However, developing such formulations for drugs with a bitter and burning taste, like dexketoprofen trometamol (DXKT), can be a real challenge due to issues of poor acceptability from patients [4,5].
The palatability of a drug is defined by the European Medicines Agency (EMA) as “the overall appreciation of an (often oral) medicinal product in relation to its smell, taste, aftertaste and texture (i.e., feeling in the mouth), determined by the characteristics of the active substance, the way the active substance is formulated into a finished medicinal product, and by the characteristics of the excipients”. Regulatory authorities, such as the EMA, FDA (Food and Drug Administration), and ICH (International Council of Harmonization), emphasize the importance of palatability and taste masking in drugs for children and older adults [6,7]. Poor palatability can negatively affect patient compliance and, consequently, the drug’s efficacy, especially for bitter-tasting drugs like NSAIDs and antibiotics. To improve palatability, pharmaceutical companies and researchers use various taste-masking techniques, such as mixing with sweeteners or bitterness inhibitors, or preventing the release of the unpleasant drug taste in the oral cavity by complexation, solid dispersions, or film coatings [8,9].
The latter technique is considered an efficient method for masking bitter tastes by hindering the release of the unpleasant drug taste in the mouth, so far as it allows to meet the drug release requirement for the given dosage form, i.e., for example, the rapid release in the stomach [10]. Coatings can be obtained with different techniques, like fluid bed coating (FBC) and high-shear coating (HSC).
FBC is the most used technique for this purpose, and the applied coating may be a polymer (film coating) or a lipid (hot melt coating). In the first case, the coating agent is dissolved or suspended in a solvent and then sprayed into the powder bed, forming a film on the particle’s surface; then, the solvent evaporates, and it is removed with the fluidizing air stream [11,12]. However, considering the high water solubility of DXKT, the polymer coating would be particularly challenging in the water medium; on the other hand, it is preferable, when possible, to avoid the use of organic solvents for safety and quality reasons. In the second case, the coating agent is applied in its molten state, and it is sprayed into the powder bed without the addition of any solvent and directly solidifies on the surface of the particles. This process is quick and does not require a drying step [13,14]. However, this method is not easily used in the pharmaceutical industry, mostly due to the complexity of the equipment for the heating and spray setup.
HSC is a new technique that allows coatings with a low-melting lipid excipient to be obtained without the use of solvents or external heating sources. In fact, it causes the coating excipient to soften because of the friction generated by the movement of the granules inside the equipment, resulting in a thermal rise, thus allowing the coating to be achieved. Overall, it is a promising method for achieving high-quality coatings with improved properties, as shown by Rosiaux et al., 2018 [15], and it is suitable for different industrial applications.
This research work focused on the use of the HSC technique to obtain granules of DXKT coated with glyceryl distearate (GDS), with the final aim of developing a taste-masked and easy-to-take pharmaceutical dosage form. The proposed technique allows the coating of the drug powder without employing a complex spray setup or an external heating source. This approach aims to simplify the lipid coating process while maintaining its effectiveness. The design of experiment (DoE) was applied to identify and optimize the most significant process parameters of the HSC technique. The effectiveness of the coating was evaluated using the dissolution test in simulated saliva [16,17].
All the coated granules were characterized for their surface area, particle size, and morphological characteristics. A taste panel test was finally performed on the best-coated samples obtained in comparison with the uncoated granular DXKT to assess the actual successful masking of the unpleasant taste of DXKT.
Download the full article as PDF here: Taste Masking of Dexketoprofen Trometamol Orally Disintegrating Granules by High-Shear Coating with Glyceryl Distearate
or read it here
Materials
Dexketoprofen trometamol (DXKT) (Lusochimica SpA, Lomagna, LC, Italy), glyceryl distearate (GDS) (Gattefossè, Saint-Priest, France), colloidal silica (Evonik, Wesseling, Germany), mannitol (Roquette Freres, Beinheim, France), and sucralose (Merk, KGaA, Darmstadt, Germany) were a generous gift from Menarini Manufacturing Logistic and Services s.r.l., Florence, Italy. All other reagents were of technical grade.
Chiarugi, I.; Biagi, D.; Nencioni, P.; Maestrelli, F.; Valleri, M.; Mura, P.A. Taste Masking of Dexketoprofen Trometamol Orally Disintegrating Granules by High-Shear Coating with Glyceryl Distearate. Pharmaceutics 2024, 16, 165. https://doi.org/10.3390/pharmaceutics16020165
Read more on Orally Disintegrating Tablets (ODTs) here:


