Leveraging novel innovative thermoresponsive polymers in microneedles for targeted intradermal deposition
Abstract
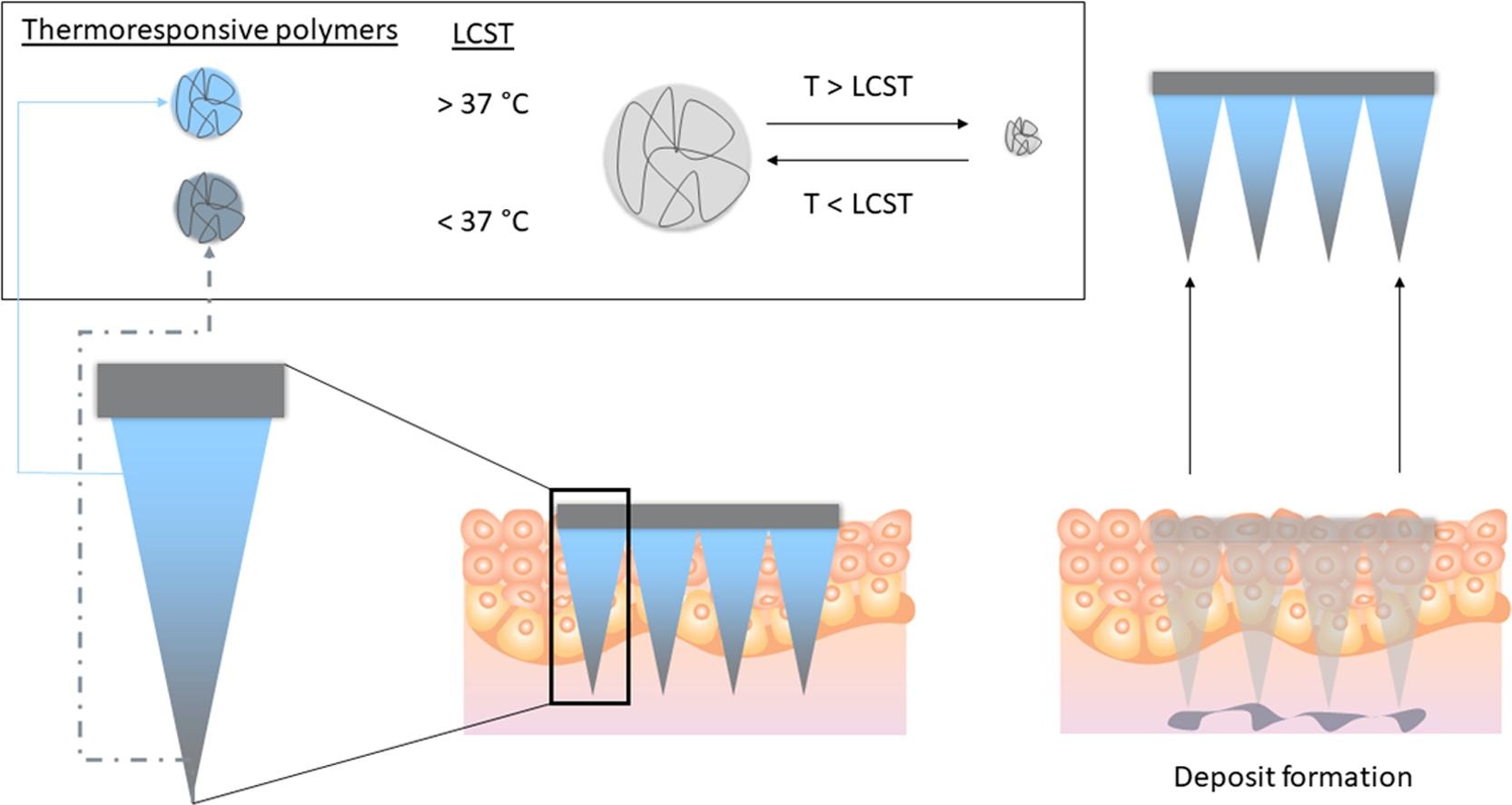
Microneedles have garnered considerable attention over the years as a versatile pharmaceutical platform that could be leveraged to deliver drugs into and across the skin. In the current work, poly (N-isopropylacrylamide) (PNIPAm) is synthesized and characterized as a novel material for the development of a physiologically responsive microneedle-based drug delivery system. Typically, this polymer transitions reversibly between a swell state at lower temperatures and a more hydrophobic state at higher temperatures, enabling precise drug release. This study demonstrates that dissolving microneedles patches made from PNIPAm, incorporating BIS-PNIPAm, a crosslinked polymer variant, exhibit enhanced mechanical properties, evident from a smaller height reduction in microneedle (∼10 %).
Although microneedles using PNIPAm alone were achievable, it displayed poor mechanical strength, requiring the inclusion of additional polymeric excipients like PVA to enhance mechanical properties. In addition, the incorporation of a thermoresponsive polymer did not have a significant (p > 0.05) impact on the insertion properties of the needles as all formulations inserted to a similar depth of 500 µm into ex vivo skin. Furthering this, the needles were loaded with a model payload, 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DID) and the deposition of the cargo was monitored via multiphoton microscopy that showed that a deposit is formed at a depth of ≈200 µm. Also, it was revealed that crosslinked-PNIPAm (Bis-PNIPAm) formulations exhibited notable skin accumulation of the dye only after 4 h, independent of the excipient matrix used. This phenomenon was absent in non-crosslinked PNIPAm formulations, indicating a deposit formation in Bis-PNIPAm microneedle formulation. Collectively, this proof-of-concept study has advanced our understanding on the possibility to use PNIPAm for dissolving microneedle fabrication which could be harnessed for the deposition of nanoparticles into the dermis, for extended drug release within the skin.
Introduction
Due to the large area and accessibility of the skin, this organ has been explored as a viable route for the administration of therapeutics. However, methods such as intradermal and transdermal delivery vary in their level of invasiveness. Within contrast to an oral-based drug delivery system, intradermal and transdermal formulation obviates the issues of gastric irritation, and first-pass metabolism. (Alkilani et al., 2015) Fundamentally, intradermal and transdermal drug delivery systems are limited by the skin’s tough outermost layer, the stratum corneum (SC). The SC only allows relatively lipophilic compounds with a molecular weight smaller than 500 Da to diffuse into the skin, limiting the utility of this route of administration for drug delivery. (Scheuplein and Blank, 1971); (Tiwari et al., 2022).
Over the decades, various methods have been developed, such as iontophoresis, electroporation and ultrasound in an attempt to circumvent the limitations imposed by the SC. (Malinovskaja-Gomez et al., 2016) However, these methods often require complex and bulky equipment that limits the translational value of these approaches for clinical use. (Alexander and Dwivedi, 2012) The emergence of microneedles has been highlighted as an interesting alternative to penetrate the SC in a painless, blood-free fashion way while expanding the range of delivered therapeutics. Dissolving microneedle is one of the most commonly used class of microneedle employed for the delivery of a range of therapeutic in intradermal delivery due to the ease of fabrication whilst endowing stability of the loaded therapeutic. (Sabri et al., 2019) Dissolving microneedles are considered one of the most effective approaches, as they can incorporate the drug directly into the dissolvable polymer matrix, leading to the release of the drug into the surrounding dermal tissue upon in-skin dissolution. (Sabri et al., 2020)
The most common materials used for fabricating the dissolving microneedles included sugars (sucrose, maltose, galactose) (Donnelly et al., 2009) and synthetic polymers like poly(vinyl alcohol, PVA) (Nguyen et al., 2018), poly(vinyl pyrrolidone, PVP) (Sabri et al., 2020) and hyaluronic acid. (Kang et al., 2022) As these excipients are categorized as Generally Recognized as Safe (GRAS) materials, most formulators typically employ these sugars and polymers in the fabrication of microneedles with more emphasis being placed on the payload and the design of the patches. This is because the use of established ingredients would most likely obviate the need for rigorous trials in order to bring a potential microneedle formulation to the market. Nevertheless, as our understanding of disease pathophysiology has advanced over the years, it has come to light that there is a need to develop intelligent pharmaceutical system which could respond accordingly to physiological stimuli in order to deliver the payload in an elegant and targeted way. Nevertheless, to achieve such goals, formulators ought to adapt a different paradigm shift in the choice of excipient used in designing microneedle system with an emphasis on using smart polymers with bio-responsive properties.
Recently, stimuli-responsive microneedles, typically based on a polymer matrix, have been introduced to control the release of the payload. (Jamaledin et al., 2020) Stimuli-responsive materials included a wide range of compounds that are capable of responding to change in their surrounding environment. These systems release payloads in response to physiological signals as internal stimuli (pH, redox, enzymes) or external stimuli (temperature, electric field, magnetic signals). (Rejinold et al., 2016, Hardy et al., 2016, Cuggino et al., 2019) Thermoresponsive polymers have garnered considerable interest in the field of drug delivery based on their ability to exhibit conformational change, (Tiwari et al., 2019); (Witting et al., 2015) especially at physiologically-relevant temperature. Poly (N-isopropylacrylamide), (PNIPAm) is one of the most common thermoresponsive polymers used to fabricate such bio-responsive systems. (Mura et al., 2013); (Nath and Chilkoti, 2001); (Pasparakis and Vamvakaki, 2011) This polymer contains both hydrophilic amide (–CONH-) segments and hydrophobic isopropyl (–CH(CH3)2) side chains. It generally exhibits a lower critical solution temperature (LCST) at approximately 32 °C, slightly below body temperature. However, this transition temperature can be adjusted by incorporating specific hydrophobic or hydrophilic monomers through copolymerization. (Wang et al., 2016); (Kim et al., 2018) Therefore, these kinds of materials exhibit a volume phase transition, which causes a sudden change in the solvation state. These properties could be exploited by formulators to design a microneedle system, which could undergo phase transition upon skin application resulting in the formation of depot that slowly release nanoparticles overtime at the targeted skin site.
Given the biocompatibility of PNIPAm copolymers and their ever-growing use in a range of drug delivery systems, (Lima et al., 2016, Capella et al., 2019, Chen et al., 2021, Li et al., 2011) we attempt to make dissolving microneedles using this polymer. The aim is to obtain a patch that could potentially allow accumulation of the compound into the skin, giving an interesting alternative for dermatological condition. Recently, Li et al. have reported the use of grafted gelatin with carboxylic end-capped PNIPAm as a matrix for hydrogel-forming microneedles to control the release of insulin. (Li et al., 2022) However, to date, no dissolving microneedles containing PNIPAm have been reported. In this study, we combined the chemistry of PNIPAm with microneedles.
In order to achieve this goal, we have designed polymers which exhibit a transition temperature below the physiological temperature while still exhibiting the suitable mechanical properties and the swelling behavior needed to fabricate dissolving microneedles. (Kim et al., 2012, Van Gheluwe et al., 2021, Prow et al., 2011) In addition, we also explored the utility of crosslinking PNIPAm, self-assembling into nanoparticles, which could increase the mechanical strength and performance of microneedles. (Puleo et al., 2013, Rana and De la Hoz Siegler, 2021, Wang et al., 2023) This proof-of-concept study demonstrate the utility of PNIPAm-based microneedle patches as a promising delivery system that could be exploited for the targeted deposition of active compound for managing localized skin diseases.
Download the full article as PDF here: Leveraging novel innovative thermoresponsive polymers in microneedles for targeted intradermal deposition
or read it here
Materials
Dioctadecylamine (CAS #112–99-2), N,N’-dicyclocarbodiimide (DCC, CAS #538–75-0), N, N’Methylenebis(acrylamide) (BIS, CAS #110–26-9) N-isopropylacrylamine (NIPAM, CAS #2210–25-5), polyvinyl alcohol (PVA Mw 9–10 kDa 13–23 kDa and 31–50 kDa 89 % hydrolysed, CAS #9002–89-5), 2,2- Azobis(2-methylpropionitrile) (AIBN, CAS #78–67-1), phosphate buffered tablets (PBS), polyvinylpyrrolidone (PVP Mw 90 kDa, CAS #9003–39-8), sodium dodecyl sulfate (CAS #151–21-3), methacrylic acid (contains 250 ppm MEHQ as inhibitor, CAS #79–41-4) and glycerol (CAS #56–81-5) were bought from Sigma Aldrich (St. Louis, MO, USA or Dorset, U.K). The 4-cyano-4-(dodecylsulfanylthiocarbonyl) sulfanyl pentanoic acid (CAS #870196–80-8) was ordered from Strem Chemical (Newburyport, MA, USA). The flash silica gel for chromatography was purchased from Silicycle (Quebec, Canada). 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindodicarbocyanine perchlorate (DID) was purchased from Fisher Scientific (Waltham, MA, USA). PVP with a Mw of 58 kDa was donated by Ashland chemicals (Kidderminster, U.K). Ammonium persulfate (APS, CAS #7727–54-0) was purchased from Alfa Aesar (Massachuset, USA). Full-thickness porcine skin was obtained from a stillborn piglet less than 24 h post-mortem and stored in −20 °C until use.
Sabrina Roussel, Jakes Udabe, Akmal Bin Sabri, Marcelo Calderón, Ryan Donnelly, Leveraging novel innovative thermoresponsive polymers in microneedles for targeted intradermal deposition, International Journal of Pharmaceutics, Volume 652, 2024, 123847, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123847.
Read more articles on microneedles from this research group :
- Fabrication of dissolving microneedles for transdermal delivery of protein and peptide drugs
- Microneedles for advanced ocular drug delivery
- Microneedle array-based electrochemical sensor functionalized with SWCNTs for the highly sensitive monitoring of MDMA in interstitial fluid