Do we need to replace Titanium Dioxide in Pharmaceutical Formulations?
Update from October 7th: News on EU commission’s decision:
On what have the member states voted on 7 October and what are the next steps?
“The Member States have expressed a favourable opinion, in the context of a Standing Committee on Plants, Animals, Food and Feed, on a proposal by the European Commission to withdraw the authorisation to use a food additive (Titanium dioxide /Ti02 also known as E171) in food products.
In the coming weeks, the Council and the European Parliament will have the possibility to give feedback on the draft Regulation during what is called the “scrutiny period” (two months to consider whether there are any grounds to object to the draft Regulation).
The Commission services will also inform the WTO members on the draft Regulation through the SPS notification system.”
To properly address this important topic, we decided to host a panel discussion on Titanium Dioxide at ExciPerience 2022. The experts Dr. Mahmud Yunis, Dave Schoneker, Dr. David Lockley and Dr. Jason Teckoe joined their host Philippe Tschopp to talk about the future use of E171.
Click here to watch the panel
What can we expect the regulation to enter into force?
“If there is no objection from the Council or the Parliament, the Regulation is expected to be adopted and published at the beginning of 2022.”
Titanium dioxide is also used in medicines. What is foreseen for medicines containing E171?
“In line with the applicable legislation and on the basis of the European Medicines Agency (EMA) analysis on the use of titanium dioxide in medicines published 8 October 2021, the Regulation foresees that titanium dioxide remains for the time being on the list of authorised additives to allow its use in medicinal products as a colour. One of the reasons for that decision is to avoid shortages of medicinal products containing the colourant as this could impact public health, animal health and welfare. The replacement of titanium dioxide will also require investigation and testing of suitable alternatives to ensure that quality, safety and efficacy of medicines is not affected negatively.
A review clause is foreseen in the draft Regulation according to which the Commission is to re-evaluate the situation within 3 years after the date of entering into force of the Regulation, on the basis of an updated assessment by the EMA.
This gives a clear sign that the pharmaceutical industry should make any possible efforts to accelerate the research and development of alternatives to replace titanium dioxide in both new and already authorised products, and to submit the necessary changes to the terms of the marketing authorisations concerned.
More information:
- Final feedback from European Medicine Agency (EMA) to the EU Commission request to evaluate the impact of the removal of Titanium dioxide from the list of authorised food additives on medicinal products
- Annex I – Use of titanium dioxide as excipient in human medicines – Industry feedback to QWP Experts / EMA questions
- Annex 2 – Use of titanium dioxide as excipient in veterinary medicines – Industry feedback to QWP Experts / EMA questions”
Find more Q+A from the european commission here
Titanium dioxide has been under discussion for some time now. In 2019, France made the decision to completely ban TiO2 in the nutraceutical sector from January 1, 2020. Now, the EFSA panel concluded, that E171 (TiO2) can no longer be considered as safe when used as a food additive in Europe. Besides its use in the food industry, TiO2 is also used in many pharmaceutical formulations, e.g. mainly in coatings and in hard capsules for opacity. For this reason we decided to do a small first LinkedIn Poll regarding the future of TiO2 in the pharmaceutical sector. We got very interesting results with a certain direction, and several detailed industry expert comments:
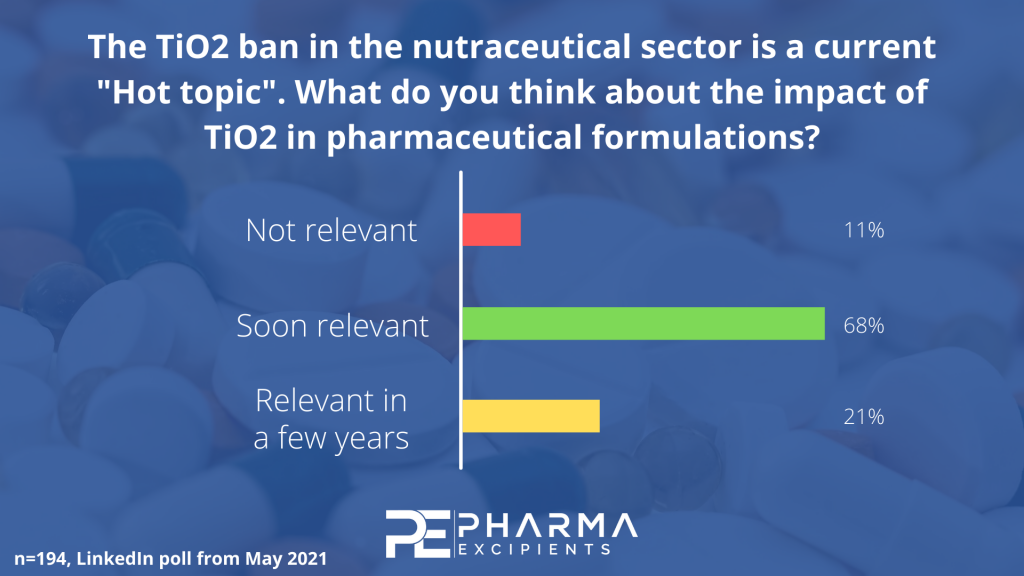
As you can see, nearly two hundred excipients industry specialists participated in our small poll. Most of the respondents (68%) think that TiO2 or the ban of TiO2 will become a very important topic in the pharmaceutical sector, already in a short time. Another 21% think it will become a very relevant topic, but in a few years. Nevertheless, there are 11% of industry specialists that have the opinion that it is not relevant at all.
In addition to the poll participation, we were sent comments and assessments from many different excipient experts and companies, which we want to give an overview of below.

One expert noted that many companies are already reformulating their products without TiO2. Due to the current safety concerns, this has been occurring more recently for several projects of his company. Even before that, many products were manufactured without TiO2 at the specific request of customers according to this poll participant. Especially in the nutraceutical sector, reformulations of products without TiO2 are increasing, and the EFSA decision reinforced this trend.
However there were also commentators that pointed out that it is difficult to say whether TiO2 should really be banned in the pharmaceutical sector. After all, according to their opinion titanium dioxide is a very important ingredient in thousands of today’s formulations, e.g. as a coating ingredient or to ensure the opacity of hard capsules.
If existing pharmaceutical product formulations are changed, these reformulated products would in turn have to be re-validated and re-verified for all possible properties because of variation submissions. According to another expert, it is questionable whether the responsible authorities can do this with this volume of products without bottlenecks (elsewhere).
On the other hand, one expert argued that TiO2 can be replaced quite “easily” by calcium carbonates or magnesium oxides. A visual difference is not visible to the naked eye anyway in the opinion of the commentator.
Another poll participant raised the question, if titanium dioxide should now really be banned, how to deal with other products regarding possible nanoparticles. He named excipients like magnesium stearate, silicon oxide or microcrystalline cellulose, which are also in discussion. If they would also be banned in a similar process, the pharmaceutical industry could face major difficulties.
The topic of the necessity of a full ban of TiO2 in pharmaceutical formulations remains controversial, even a majority of our poll participants think the developments go in this direction in a relatively short time. So the topic will be with us for the near future. It will be interesting to see how the regulating authorities in the various countries go forward here. Depending on their decisions and the regulatory framework, the pharmaceutical industry will be faced with major challenges.
Titanium dioxide is also no longer considered safe as feed additive

