In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin
The external use of curcumin is rare, although it can be a valuable active ingredient in the treatment of certain inflammatory diseases. The aim of our experimental work was to formulate topical dosage forms containing curcumin for the treatment of atopic dermatitis. Curcumin has extremely poor solubility and bioavailability, so we have tried to increase it with the usage of self-emulsifying drug delivery systems. Creams and gels were formulated using penetration-enhancing surfactants and gelling agents. The release of the drug from the vehicle and its penetration through the membrane were determined using a Franz diffusion cell. An MTT cytotoxicity and in vitro antioxidant assays were performed on HaCaT cell line.
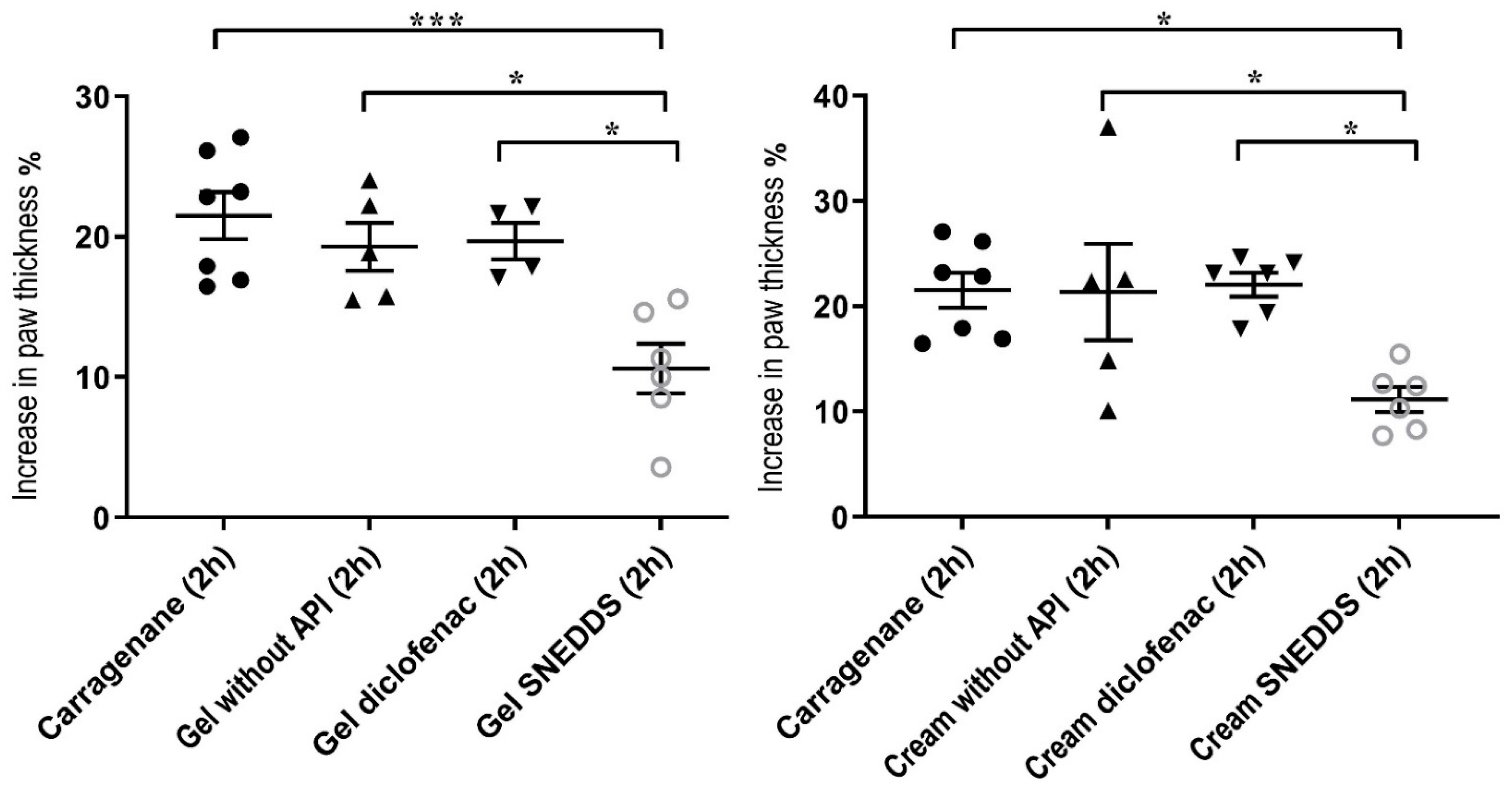
The in vivo anti-inflammatory effect of the preparations was tested by measuring rat paw edema. In addition, we examined the degree of inflammation induced by UV radiation after pretreatment with the cream and the gel on rats. For the gels containing SNEDDS, the highest penetration was measured after half an hour, while for the cream, it took one hour to reach the maximum concentration. The gel containing Pemulen TR-1 showed the highest drug release. It was determined that the curcumin-containing preparations can be safely applied on the skin and have antioxidant effects. The animal experiments have proven the effectiveness of curcumin-containing topical preparations.
Download the full article as PDF here In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin
or read it here
Materials
Labrasol (caprylocaproyl polyoxyl-8 glyceride), Tefose 63 (mixture of PEG-6 stearate, ethylene glycol palmitostearate and PEG-32 stearate), and Sedefos 75 (mixture of triceteareth-4 phosphate, ethylene and diethylene glycol stearate) were obtained from Gattefossé (Lyon, France). Cremophor RH 40 (polyoxyl 40 hydrogenated castor oil) was obtained from BASF Company (Ludwigshafen, Germany). Pemulen TR-1 and Carbopol 974P were purchased from Lubrizol Corporation, Ohio, USA. Stearic acid, cetylstearyl alcohol, propylene glycol, and isopropyl myristate were purchased from Hungaropharma Ltd., (Budapest, Hungary). The MTT dye, curcumin, DPPH free radical, human and rat TNF-α and IL-1β ELISA Kits and ingredients needed for the cell maintenance were purchased from Sigma-Aldrich (Budapest, Hungary). The immortalized human keratinocyte cell line (HaCaT) was obtained from Cell Lines Service (CLS, Heidelberg, Germany).
Frei, G.; Haimhoffer, Á.; Csapó, E.; Bodnár, K.; Vasvári, G.; Nemes, D.; Lekli, I.; Gyöngyösi, A.; Bácskay, I.; Fehér, P.; et al. In Vitro and In Vivo Efficacy of Topical Dosage Forms Containing Self-Nanoemulsifying Drug Delivery System Loaded with Curcumin. Pharmaceutics 2023, 15, 2054. https://doi.org/10.3390/pharmaceutics15082054
Read more on Orally Disintegrating Tablets (ODTs) here:


