Effect of Talc and Vitamin E TPGS on Manufacturability, Stability and Release Properties of Trilaurin-based Formulations for Hot-Melt Coating
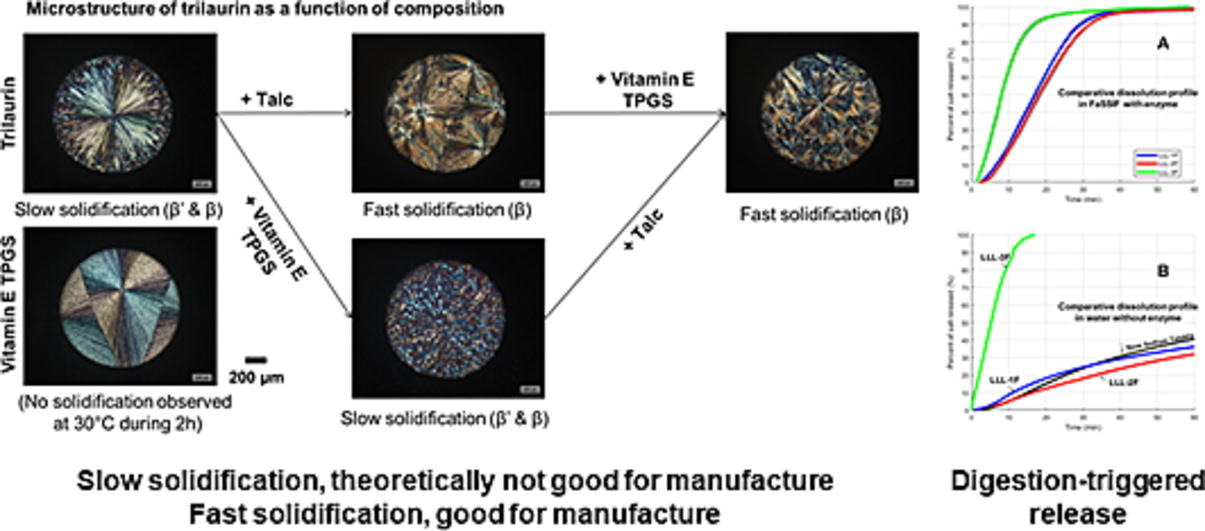
This study was focused on one particular case of hot-melt coating with trilaurin – a solid medium-chain monoacid triglyceride. The challenge of using trilaurin as coating agent in melting-based processes is linked to its relatively low melting profile: 15.6°C (Tm,α), 35.1°C (Tm,β′) and 45.7°C (Tm,β). From a process perspective, the only possibility to generate products coated with formulations composed of trilaurin is by setting thermal operational conditions above Tm,α. From a material perspective, this processing possibility depends principally on trilaurin crystallisation which was investigated via a set of analytical techniques including turbidimetry, calorimetry, hot-melt goniometry, and polarised light microscopy. A highly soluble drug model substrate (sodium chloride crystals) was coated with three selected trilaurin-based formulations: (i) trilaurin, (ii) trilaurin plus talc, and (iii) trilaurin plus vitamin E TPGS and talc. Coated salt crystals were then analysed to investigate processing performance, coating quality, stability and release properties under digestion effect.
The results show that firstly, talc addition promotes nucleation and crystal growth and, as a consequence, it facilitates the manufacture of trilaurin-based formulations. Secondly, the formulation of a solid triglyceride and a hydrophilic surfactant could potentially cause release instability, but formula (iii) was found to be stabilised by a mechanism whereby trilaurin crystallization enhanced in the presence of talc immobilised vitamin E TPGS in its crystal lattice. Thirdly, talc addition did not significantly influence trilaurin digestion which endows products with an immediate release in lipolytic conditions instead of an extended liberation in pure water. Nor did the addition of one or two additives alter the extent of trilaurin digestion under the conditions studied. These important findings relate to product manufacturability, stability, and release properties. A good understanding of material properties (e.g. crystallisation, polymorphism, digestibility) is essential for melt-processing, lipid coating stabilising and modulation of release profile of solid lipid-coated product, as demonstrated in this case study with trilaurin.
Materials
Trilaurin (LLL, Tm,α = 15.6°C, Tm,β’ = 35.1°C, Tm,β = 45.7°C) and vitamin E TPGS (VitE_TPGS, HLB ≈13) were purchased from TCI Europe N.V. and PMC Isochem (France). Luzenac PHARMA M (talc, T) (d50,laser = 10.5 µm) was kindly donated by Azelis (Courbevoie, France). Bile extract porcine (B8631-100G), cholate sodium (C24H39NaO5.xH2O) (C1254-100G), deoxycholate sodium (C24H39NaO4) (D6750-25G), 4-bromophenyl-boronic acid (B75956-5G), tris-maleate (NH2C(CH2OH)3.C4H4O4) (T3128-50G), calcium chloride
Read more
Van-Trung-Tin Huynh, Suenia de Paiva Lacerda, Fabienne Espitalier, Eric Beyssac, Maria-Inês Ré,
Effect of Talc and Vitamin E TPGS on Manufacturability, Stability and Release Properties of Trilaurin-based Formulations for Hot-Melt Coating, International Journal of Pharmaceutics, 2024, 123866, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2024.123866.

