Transdermal delivery of acemetacin loaded microemulsions: preparation, characterization, in vitro – ex vivo evaluation and in vivo analgesic and anti-inflammatory efficacy
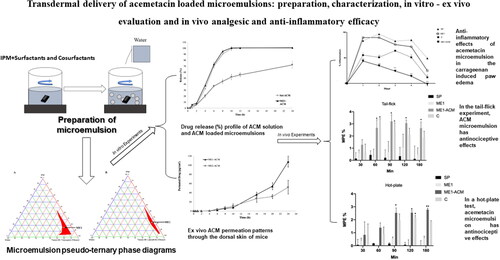
The goal of this study was to see if microemulsions might be used as a transdermal drug delivery system for acemetacin (ACM). Microemulsions containing isopropyl myristate, Tween 80, Transcutol HP, Labrafil M1944 CS, ethanol, and distilled water were used to create pseudo-ternary phase diagrams. In microemulsion systems, the ultimate concentration of ACM was 1% (w/w). Conductivity, droplet size, zeta potential, polydispersity index, viscosity, and pH were used to characterize the microemulsions. Furthermore, in vitro permeability investigations were carried out utilizing diffusion cells derived from the dorsal skin of mice.
The results of characterization studies such as droplet size, polydispersity index, zeta potential, conductivity values of ideal formulation were found 9.107 ± 1.588 nm, 0.283 ± 0.062, −0.313 ± 0.006 mV, 0.018 ± 0.012 µS/cm, respectively. The penetration of ACM from ideal formulation was greater than the other developed formulations. The anti-inflammatory effect of ACM was evaluated in mice using the paw edema test. In addition, all test microemulsions had a significant analgesic effect in the hot plate and tail-flick tests as compared to the control. Finally, microemulsion formulations might be used to deliver ACM transdermally.
Read more
(2023) Transdermal delivery of acemetacin loaded microemulsions: preparation, characterization, in vitro – ex vivo evaluation and in vivo analgesic and anti-inflammatory efficacy, Journal of Dispersion Science and Technology, DOI: 10.1080/01932691.2023.2175691

