Application of Design Space, Uncertainty, and Risk Profile Strategies to the Development and Validation of UPLC Method for the Characterization of Four Authorized Phosphodiesterase Type 5 Inhibitors to Combat Counterfeit Drugs
Background: Counterfeit medicines are an increasing scourge that are difficult to identify and they have become industrialized and widespread through highly organized illegal channels.
Objective: This research aims to develop a robust method to determine four phosphodiesterase type-5 inhibitors in counterfeit drugs based on ultra-performance liquid chromatography.
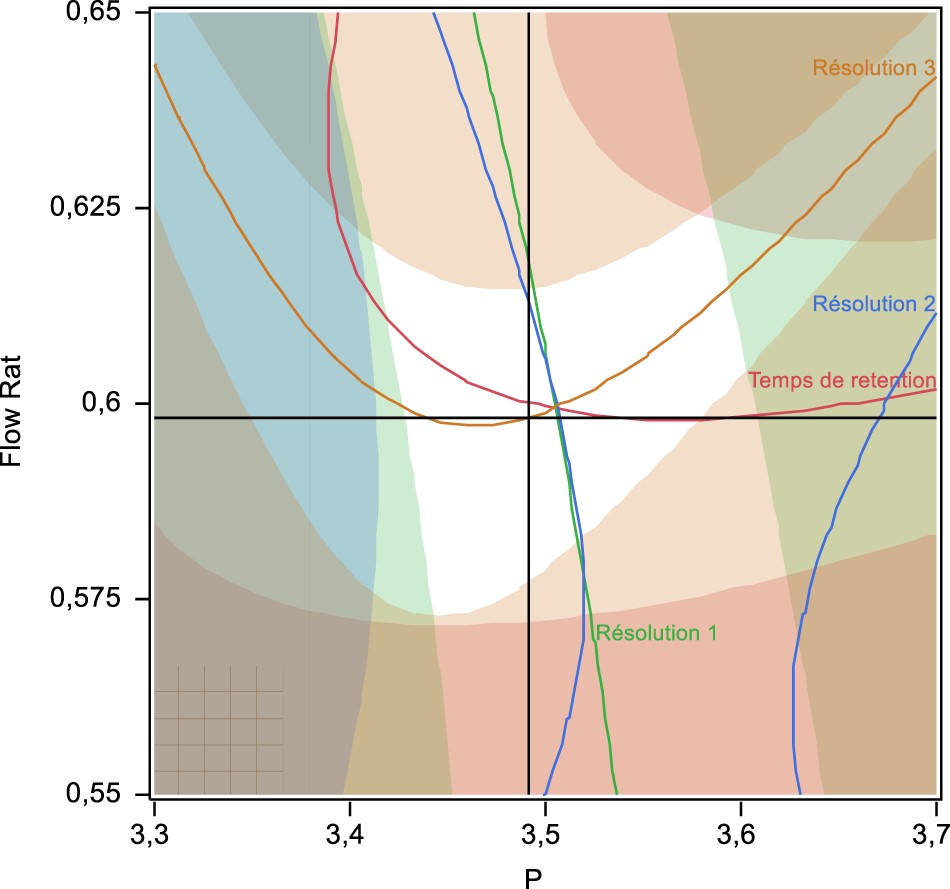
Method: Experimental design methodology (DOE) and design space (DS) recommended by ICH Q8 were used side-by-side in the development phase to define the optimal parameters as well as the robustness of the chromatographic method. Moreover, both the uncertainty and risk profile derived from the β-content and γ-confidence tolerance interval were investigated during the validation phase to examine the performance of this method.
Results: Successful chromatographic results, in a high resolution between the four active ingredients and an optimal analysis time of less than 1.6 min, were achieved at the end of the optimization phase. In addition, validation results show a low risk of future measurements outside acceptance limits set at 5%.
Conclusions: Our procedure was successfully applied in the routine phase to identify 23 illicit formulations of an erectile dysfunction drug.
Highlights: An efficient method for the characterization of 4 authorized phosphodiesterase in less than 1.6 min was established. A DS approach was applied to test the performance of this analytical method during analytical development. A risk profile was then carried out to approve the validity of the analytical method through the uncertainty profile approach.
Materials: Reference standards of udenafil, tadalafil, sildenafil citrate, and vardenafil were provided by the National Laboratory of Medicines Control (LNCM). Acetonitrile (HPLC grade), formic acid (98%), and ammonium formate were supplied by LNCM. The matrix used in the validation phase was comprised of functional excipients to assimilate the required properties of the pharmaceutical form. It contains the following compounds: corn starch, sodium lauryl sulphate, colloidal silicon dioxide, lactose, hydroxypropyl cellulose, talc, magnesium stearate, microcrystalline cellulose, croscarmellose sodium, and Instacoat.
Download the full article as a PDF here or read it here
Article information: Yassine Hameda Benchekroun, Miloud El Karbane, Bouchaib Ihssane, Hasnaa Haidara, Mohamed Azougagh, Taoufiq Saffaj, Application of Design Space, Uncertainty, and Risk Profile Strategies to the Development and Validation of UPLC Method for the Characterization of Four Authorized Phosphodiesterase Type 5 Inhibitors to Combat Counterfeit Drugs, Journal of AOAC INTERNATIONAL, Volume 103, Issue 3, May-June 2020, Pages 715–724, https://doi.org/10.1093/jaocint/qsz006
Read more on Talc here:


