Polymeric and Lipid Nanoparticles: Which Applications in Pediatrics?
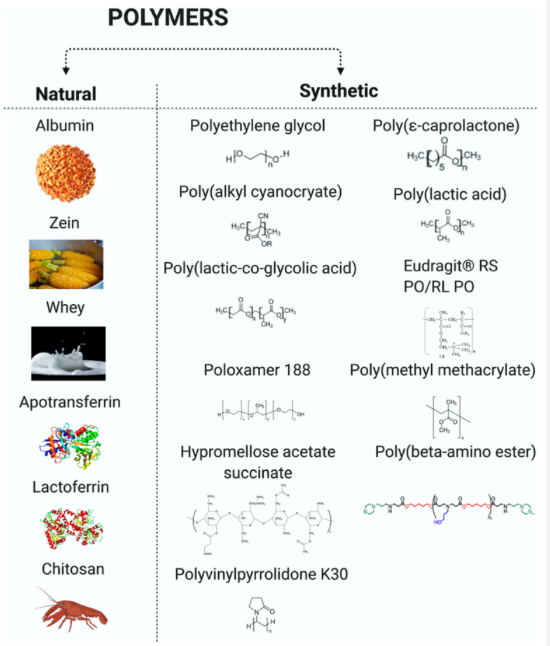
Natural and synthetic polymer used to make pediatric nanoparticles (created with Biorender)
Natural Polymers: Albumin, Zein, Whey, Apotransferrin, Lactoferrin, Chitosan
Synthetic Polymers: Polyethylene Glycol, Poly(alkyl cyanoceyate), Poly(lactic-co-glycolic acid), Poloxamer 188, Hypromellose acetate succinate, Polyvinlypryrrodidone K30, Poly(e-caprolactone), Poly(lactic acid) Eudragit RS PO/RL PO, Poly(methyl mathacrylate), Poly(beta-amino ester)
Conclusions
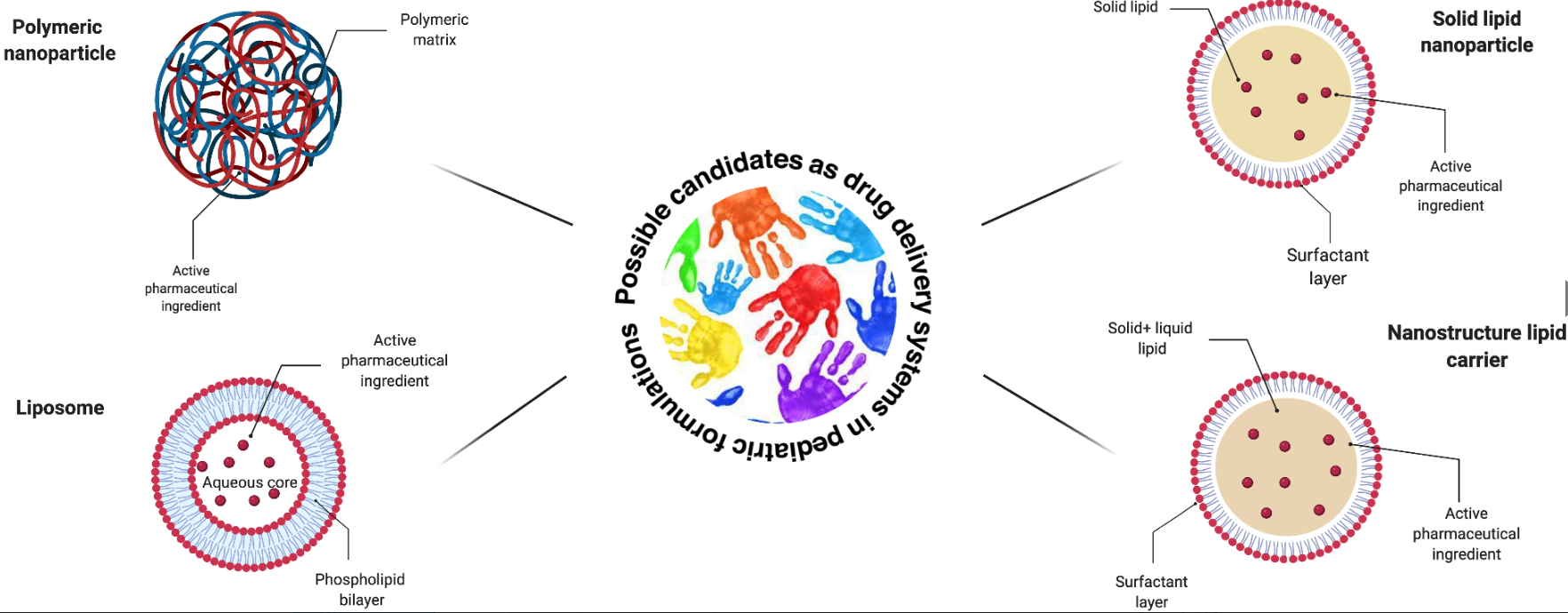
Polymeric and lipid NPs have been widely studied to treat pediatric diseases, but only a few are used in therapy and only to treat pediatric cancers. Great attention is paid to the excipients used, to the safe and easy-to-scale-up preparation methods and, of course, to the size in order to obtain safe carriers.