Direct Powder Extrusion of Paracetamol Loaded Mixtures for 3D Printed Pharmaceutics for Personalized Medicine via Low Temperature Thermal Processing
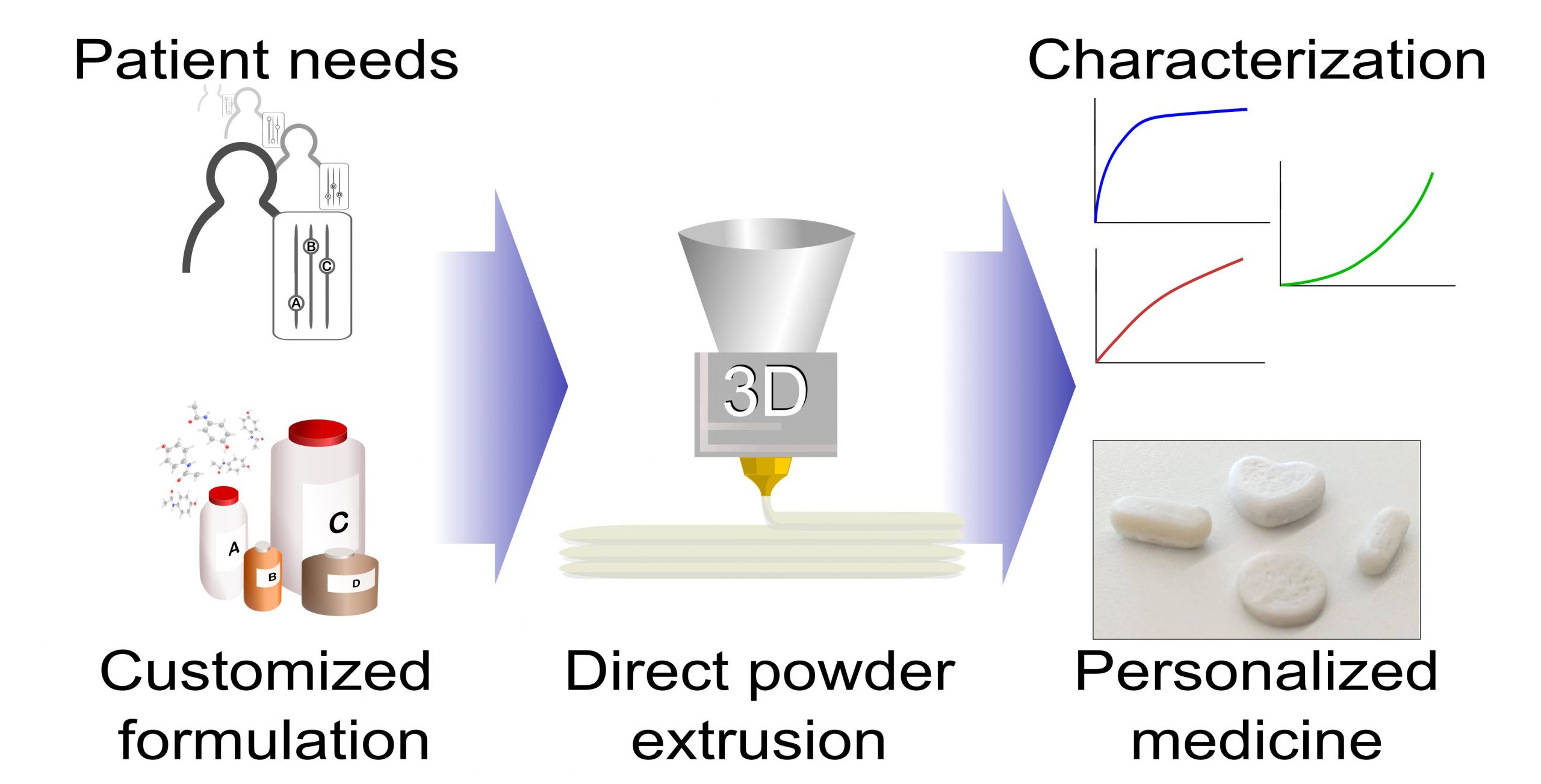
Three-dimensional printed drug development is nowadays an active area in the pharmaceutical industry, where the search for an appropriate edible carrier that permits the thermal processing of the mixture at temperature levels that are safe for the drug is an important field of study. Here, potato starch and hydroxypropyl cellulose based mixtures loaded with paracetamol up to 50% in weight were processed by hot melt extrusion at 85 °C to test their suitability to be thermally processed.
The extruded mixtures were tested by liquid chromatography to analyze their release curves and were thermally characterized. The drug recovery was observed to be highly dependent on the initial moisture level of the mixture, the samples being prepared with an addition of water at a ratio of 3% in weight proportional to the starch amount, highly soluble and easy to extrude. The release curves showed a slow and steady drug liberation compared to a commercially available paracetamol tablet, reaching the 100% of recovery at 60 min.
The samples aged for 6 weeks showed slower drug release curves compared to fresh samples, this effect being attributable to the loss of moisture. The paracetamol loaded mixture in powder form was used to print pills with different sizes and geometries in a fused deposition modelling three-dimensional printer modified with a commercially available powder extrusion head, showing the potential of this formulation for use in personalized medicine.
Download the full article as a PDF here or read it here
Introduction
Mendibil, X.; Tena, G.; Duque, A.; Uranga, N.; Campanero, M.Á.; Alonso, J. Direct Powder Extrusion of Paracetamol Loaded Mixtures for 3D Printed Pharmaceutics for Personalized Medicine via Low Temperature Thermal Processing. Pharmaceutics 2021, 13, 907. https://www.mdpi.com/1999-4923/13/6/907/htm

