New and Novel Excipients – Part 2

A week ago I wrote about the importance of new and novel excipients for the pharmaceutical industry and presented Klucel™ xtend and BioSustane™ in last week´s edition of “The Excipients Week”.
In the meantime I launched a small poll with the title “Was there innovation in excipients over the last 2-3 years?” It did not get a lot of votes but still 70% share the opinion that something was moving and several excipient manufacturers also informed about their latest developments which I would like to share with you. There are even some CPHI Award finalists!
Emulfree® Duo
Emulfree® Duo by Gattefossé is a ready-to-use PEG-free system. It has been designed to stabilize the oil phase within the bi-gel, by ensuring fine and homogeneous dispersion in the aqueous phase. With Emulfree® Duo the whole process can be carried out at room temperature, which is ideal for thermo sensitive drugs. This excipient is compatible with a wide range of oils, solvents and penetration enhancers, and enables the formulation of a wide range of textures, from light lotions to rich creams, depending on the overall formulation
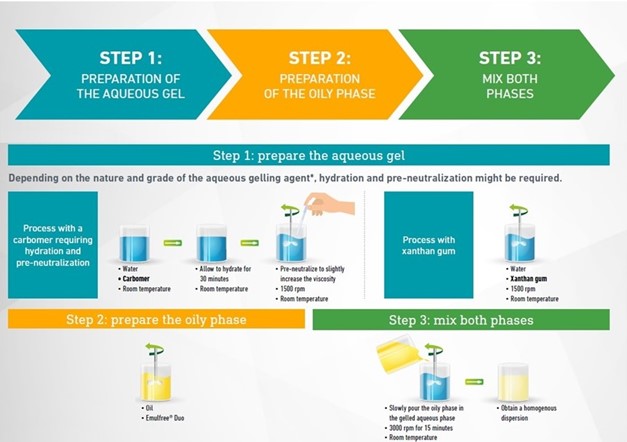
More information on Emulfree® Duo
Kollitab™ DC 87 L – BASF´s new all-in-one tableting solution

Your new all-in-one tableting solution with filler, binder, disintegrant and lubricant: Kollitab™ DC 87 L
Kollitab™ DC 87 Lby BASF is an all-in-one tableting solution that includes a filler, binder, disintegrant and lubricant.
- It provides superior flowability, achieves high tablet strength when applying a broad range of compression forces (both low and high), and ensures fast tablet disintegration.
- Its “all-in-one” nature enables fast tablet development, simplifies formulation processes, and reduces manufacturing complexity.
More information on Kollitab™ DC 87 L
EUDRACAP®
EUDRACAP® by Evonik are ready-to-fill coated capsules for fast drug development. EUDRACAP® pre-locked capsules are suitable for the filling of drug forms including powders, pellets and granules. They are easy to open and close on standard capsule filling systems.

An increasing number of active ingredients are also sensitive to heat, moisture, gastric acids or certain processing conditions. Here, EUDRACAP® can help protect the active ingredient to optimize rates of absorption and avoid premature dissolution. Together with the regulatory excellence of EUDRAGIT® and Evonik’s local market knowhow, these USP and EP compliant EUDRACAP® capsules can help you to reduce clinical risk and accelerate time to market.
THE BENEFITS OF EUDRACAP®
- Precise pH targeting
- Effective acid resistance for up to 4 hours
- Optimized absorption rates
- Reduce clinical risk
- Accelerate time to market
Apisolex™
Apisolex™ by Lubrizol is a Solubility-enhancing Excipient for Injectable Drug Formulations.
Approximately 60% of potential active pharmaceutical ingredients (APIs) under development, and more than 40% of those in reformulation, are poorly water soluble. Excipients offer the most versatile approach to solving these solubility challenges and have long played a key role in allowing poorly soluble APIs to be formulated into products with improved bioavailability and therapeutic effects. However, as the number of poorly soluble APIs grows, existing excipients are still not able to solve all of the complex formulation challenges.
Rescue Your Parenteral Drug Formulation with Apisolex™ Polymer
- Safe, poly-amino acid based amphiphilic polymer which is a non-toxic, non-immunogenic, biocompatible, and biodegradable alternative to PEG.
- Increases solubility of hydrophobic APIs by up to 50,000-fold.
- High drug loading (up to 40:100 API:solubilizer ratio).
- Forms stable, lyophilized drug product that reconstitutes in less than 30 seconds in saline.
- Enables IP protection and 505(b)(2) formulations/lifecycle management.
Apinovex™
Apinovex™ by Lubrizol is a Solubility-Enhancing Excipients for Amorphous Solid Dispersions.
 Lubrizol’s Apinovex™ polymers are high molecular weight polyacrylic acid excipients designed to provide both processing and formulation benefits for spray-dried amorphous solid dispersions (ASDs). Apinovex polymers enable formulators to enhance the solubility of BCS Class II and IV APIs and develop more efficient oral dosage forms with high, stable drug loading.
Lubrizol’s Apinovex™ polymers are high molecular weight polyacrylic acid excipients designed to provide both processing and formulation benefits for spray-dried amorphous solid dispersions (ASDs). Apinovex polymers enable formulators to enhance the solubility of BCS Class II and IV APIs and develop more efficient oral dosage forms with high, stable drug loading.
Enhance Oral Solubility with Apinovex™ Polymers
- High, stable drug loading (up to 80%) via spray drying.
- Significantly improved drug release profile compared to crystalline APIs.
- Enables IP protection and 505(b)(2) formulations/lifecycle management.
Parteck® COAT
Parteck® COAT by Merck is a polyvinyl alcohol (PVA) with a unique structure specifically designed for immediate-release film coating applications. Due to its optimized particle size, it helps to reduce dissolving times when preparing the coating solution, thus increasing process efficiency. Its low viscosity and high stability against moisture and oxidation makes it very well-suited for use as a film-forming polymer in coatings.

Benefits of Parteck® COAT:
- Excellent surface finishing – increase the value of your formulation
- Rapid preparation of coating liquids even at room temperature
- Low viscosity at high polymer concentration – enables reduced process times and enhanced efficiency
- Stable moisture and oxygen barrier – effective protection of moisture-sensitive APIs
- Multi-compendial material, a GRAS polymer and compliant with Ph Eur, ChP, JPE and USP

