Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive In Situ Gel – A Novel Intravesical Therapy Tackling Bladder Cancer
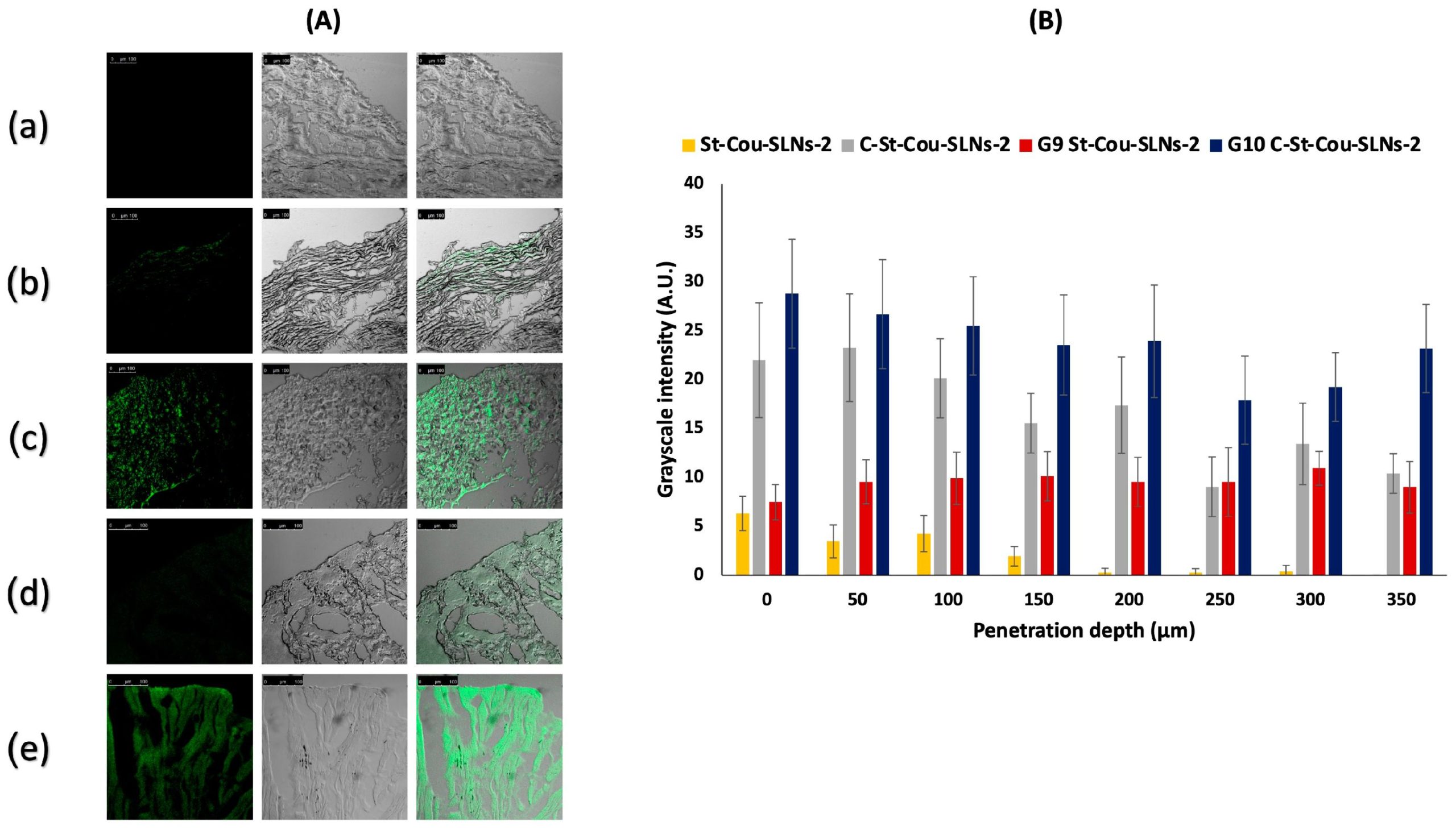
The study aim was to develop an intravesical delivery system of quercetin for bladder cancer management in order to improve drug efficacy, attain a controlled release profile and extend the residence time inside the bladder. Either uncoated or chitosan coated quercetin-loaded solid lipid nanoparticles (SLNs) were prepared and evaluated in terms of colloidal, morphological and thermal characteristics. Drug encapsulation efficiency and its release behaviour were assessed. Furthermore, cytotoxicity of SLNs on T-24 cells was evaluated. Ex vivo studies were carried out using bovine bladder mucosa. Spherical SLNs (≈250 nm) ensured good entrapment efficiencies (EE > 97%) and sustained drug release up to 142 h. Cytotoxicity profile revealed concentration-dependent toxicity recording an IC50 in the range of 1.6–8.9 μg/mL quercetin. SLNs were further dispersed in in situ hydrogels comprising poloxamer 407 (20%) with mucoadhesive polymers. In situ gels exhibited acceptable gelation temperatures (around 25 °C) and long erosion time (24–27 h). SLNs loaded gels displayed remarkably enhanced retention on bladder tissues relative to SLNs dispersions. Coated SLNs exhibited better penetration abilities compared to uncoated ones, while coated SLNs dispersed in gel (G10C-St-QCT-SLNs-2) showed the highest penetration up to 350 μm. Hence, G10C-St-QCT-SLNs-2 could be considered as a platform for intravesical quercetin delivery.
Introduction
Materials
Quercetin was obtained from M/s Sisco Research Laboratories (Maharashtra, India). Precirol was a sample gift from Gattefossé (Saint Priest, France). Stearic acid was kindly provided by Pharco Pharmaceuticals Company (Alexandria, Egypt). Poloxamer188 (P188, Pluronic-F68TM) was obtained from BASF (Ludwigshafen, Germany). Tween 80 was purchased from Chemtech (Alexandria, Egypt). Chitosan (Cs) for coating (Mw 60–120 kDa, Degree of deacetylation 85%, viscosity 27 CPS) was purchased from Sigma-Aldrich (Steinheim, Germany). Poloxamer 407 (Pluronic F127) was a sample gift from Borg Pharmaceutical Companies (Alexandria, Egypt). Carbopol 974P (Cb) was a sample gift from Lubrizol (Oevel, Belgium). Chitosan for gel preparation (Mw 161.116, degree of deacetylation 93%) was obtained from Oxford Lab Chem (Mumbai, India). Hydroxy propyl methyl cellulose (HPMC) was purchased from Cairo Pharmaceuticals Co., (Cairo, Egypt). T-24: Urinary Bladder cancer cell line (transitional cell carcinoma) was obtained from Nawah Scientific Inc., (Cairo, Egypt). Dulbecco’s Modified Eagle Medium (DMEM) high glucose, Roswell Park Memorial Institute (RPMI) 1640 high glucose, Penicillin/streptomycin, Trypsin and EDTA were purchased from Lonza GmbH (Köln, Germany). Fetal bovine serum (FBS) was purchased from Gibco (Grand Island, NY, USA). Coumarin-6 dye (cou) and Sulforhodamine B (SRB) were purchased from Sigma-Aldrich (Steinheim, Germany). Trichloroacetic acid (TCA) was purchased from Merck (Kenilworth, NJ, USA). Tris (hydroxymethyl) aminomethane (TRIS) was obtained from Chem-Lab (Zedelgem, Belgium). All other used chemicals were of analytical grade.
Shawky, S.; Makled, S.; Awaad, A.; Boraie, N. Quercetin Loaded Cationic Solid Lipid Nanoparticles in a Mucoadhesive In Situ Gel—A Novel Intravesical Therapy Tackling Bladder Cancer. Pharmaceutics 2022, 14, 2527. https://doi.org/10.3390/pharmaceutics14112527

