Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets
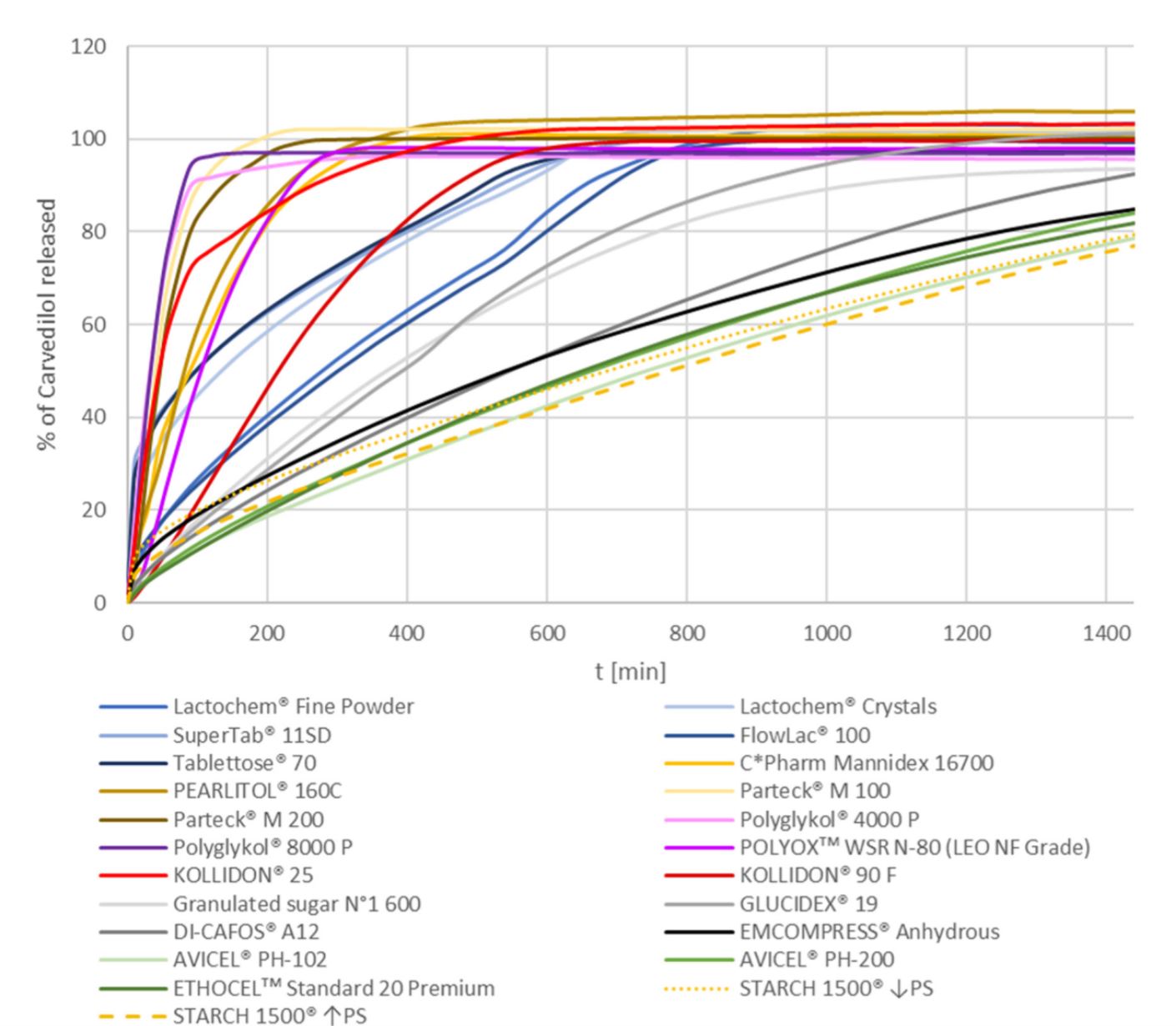
Solid dosage forms based on hypromellose (HPMC) with prolonged/extended drug release are very important from the research and industrial viewpoint. In the present research, the influence of selected excipients on carvedilol release performance from HPMC-based matrix tablets was studied. A comprehensive group of selected excipients was used within the same experimental setup, including different grades of excipients. Compression mixtures were directly compressed using constant compression speed and main compression force. LOESS modelling was used for a detailed comparison of carvedilol release profiles via estimating burst release, lag time, and times at which a certain % of carvedilol was released from the tablets. The overall similarity between obtained carvedilol release profiles was estimated using the bootstrapped similarity factor (f2). In the group of water-soluble carvedilol release modifying excipients, which produced relatively fast carvedilol release profiles, POLYOXᵀᴹ WSR N-80 and Polyglykol® 8000 P demonstrated the best carvedilol release control, and in the group of water-insoluble carvedilol release modifying excipients, which produced relatively slow carvedilol release profiles, AVICEL® PH-102 and AVICEL® PH-200 performed best.
Table 2. Excipients used as water-soluble (a) or water-insoluble (b) fillers/bulking agents/carvedilol release modifiers in experiments. The solubility of water-insoluble excipients is negligible in comparison to water-soluble ones.
| (a) | ||||
|---|---|---|---|---|
| Excipient Generic Name | Selected Excipient’s Marketed Product Name | Name Abbreviation | Manufacturer | Additional Info 1 |
| Polyethylene Glycol (Ph. Eur.: Macrogols) & Polyethylene Oxide (Ph. Eur.: Macrogols, High-Molecular-Mass) | Polyglykol® 4000 P | PEG 4k | Clariant Produkte (Deutschland) GmbH, Frankfurt, Germany | M = 4017 g/mol (CoA) |
| Polyglykol® 8000 P | PEG 8k | Clariant Produkte (Deutschland) GmbH, Frankfurt, Germany | M = 8026 g/mol (CoA) | |
| POLYOXᵀᴹ WSR N-80 (LEO NF Grade) | PEO | DUPONT, Nutrition and Biosciences (Freienbach, Switzerland) GmbH | nominal M of 200,000 g/mol; | |
| Povidone | KOLLIDON® 25 | PVP K25 | BASF SE, Ludwigshafen, Germany | K value = 24.7 (CoA) |
| KOLLIDON® 90 F | PVP K90 | BASF SE, Ludwigshafen, Germany | K value = 92.4 (CoA) | |
| Mannitol | C*Pharm Mannidex 16700 | MAN_C_1 | Cargill S.r.l., Milan, Italy | crystalline D-mannitol, sorbitol content = 0.6% (CoA) |
| PEARLITOL® 160C | MAN_C_2 | ROQUETTE Frères, Lestrem, France | crystalline D-mannitol, sorbitol content = 0.7% (CoA) | |
| Parteck® M 100 | MAN_SD_1 | Merck KGaA, Darmstadt, Germany | spray-dried D-mannitol, sorbitol content = 1.3% (CoA) | |
| Parteck® M 200 | MAN_SD_2 | Merck KGaA, Darmstadt, Germany | spray-dried D-mannitol, sorbitol content = 1.3% (CoA) | |
| Lactose Monohydrate | Lactochem® Crystals | LAC_M | DFE Pharma GmbH and Co. KG, Goch, Germany | crystalline lactose monohydrate |
| Lactochem® Fine Powder | LAC_C | DFE Pharma GmbH and Co. KG, Goch, Germany | milled lactose monohydrate | |
| SuperTab® 11SD | LAC_SD_1 | DFE Pharma GmbH and Co. KG, Goch, Germany | spray-dried lactose monohydrate | |
| FlowLac® 100 | LAC_SD_2 | MEGGLE GmbH and Co. KG, Wasserburg, Germany | spray-dried lactose monohydrate | |
| Tablettose® 70 | LAC_AG | MEGGLE GmbH and Co. KG, Wasserburg, Germany | agglomerated lactose monohydrate | |
| Sucrose | Granulated sugar N°1 600 | SUC | Tereos, Moussy-le-Vieux, France | crystalline sucrose |
| Maltodextrin | GLUCIDEX® 19 | MD_SD | ROQUETTE Frères, France | a spray-dried mixture of glucose, disaccharides, and polysaccharides |
| (b) | ||||
| Excipient Generic Name | Selected Excipient’s Marketed Product Name | Name Abbreviation | Manufacturer | Additional Info 1 |
| Anhydrous Dibasic Calcium Phosphate (Ph. Eur.: Calcium Hydrogen Phosphate) | DI-CAFOS® A12 | DCP_1 | Chemische Fabrik Budenheim KG, Budenheim, Germany | anhydrous DCP |
| EMCOMPRESS® Anhydrous | DCP_2 | JRS Pharma GmbH and Co. KG, Rosenberg, Germany | anhydrous DCP | |
| Microcrystalline Cellulose (Ph. Eur.: Cellulose, Microcrystalline) | AVICEL® PH-102 | MCC 101 | DuPont Nutrition Ireland, Little Island, Ireland | nominal particle size of app. 100 µm |
| AVICEL® PH-200 | MCC 200 | DuPont Nutrition Ireland, Little Island, Ireland | nominal particle size of app. 180 µm | |
| Ethylcellulose | ETHOCELᵀᴹ Standard 20 Premium | EC | DOW, Specialty Electronic Materials Switzerland GmbH, Horgen, Switzerland | FRC data from CoA: viscosity 20.6 mPa·s, ethoxyl content (assay) 48.7% |
| Pregelatinized Starch | STARCH 1500® sample with smaller particle size (↓PS) | PS_1 | Colorcon Inc., Harleysville, PA, USA | sieve analysis: 99.3% through 100 mesh and 46.9% through 270 mesh (CoA) |
| STARCH 1500® sample with larger particle size (↑PS) | PS_2 | Colorcon Inc., Harleysville, PA, USA | sieve analysis: 93.5% through 100 mesh and 31.9% through 270 mesh (CoA) |
Download the full article as PDF here Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets
or read it here
Ojsteršek, T.; Hudovornik, G.; Vrečer, F. Comparative Study of Selected Excipients’ Influence on Carvedilol Release from Hypromellose Matrix Tablets. Pharmaceutics 2023, 15, 1525. https://doi.org/10.3390/pharmaceutics15051525
Read more on Shellac as a pharmaceutical excipient here:


