Anti-obesity effect with reduced adverse effect of the co-administration of mini-tablets containing orlistat and mini-tablets containing xanthan gum: In vitro and in vivo evaluation
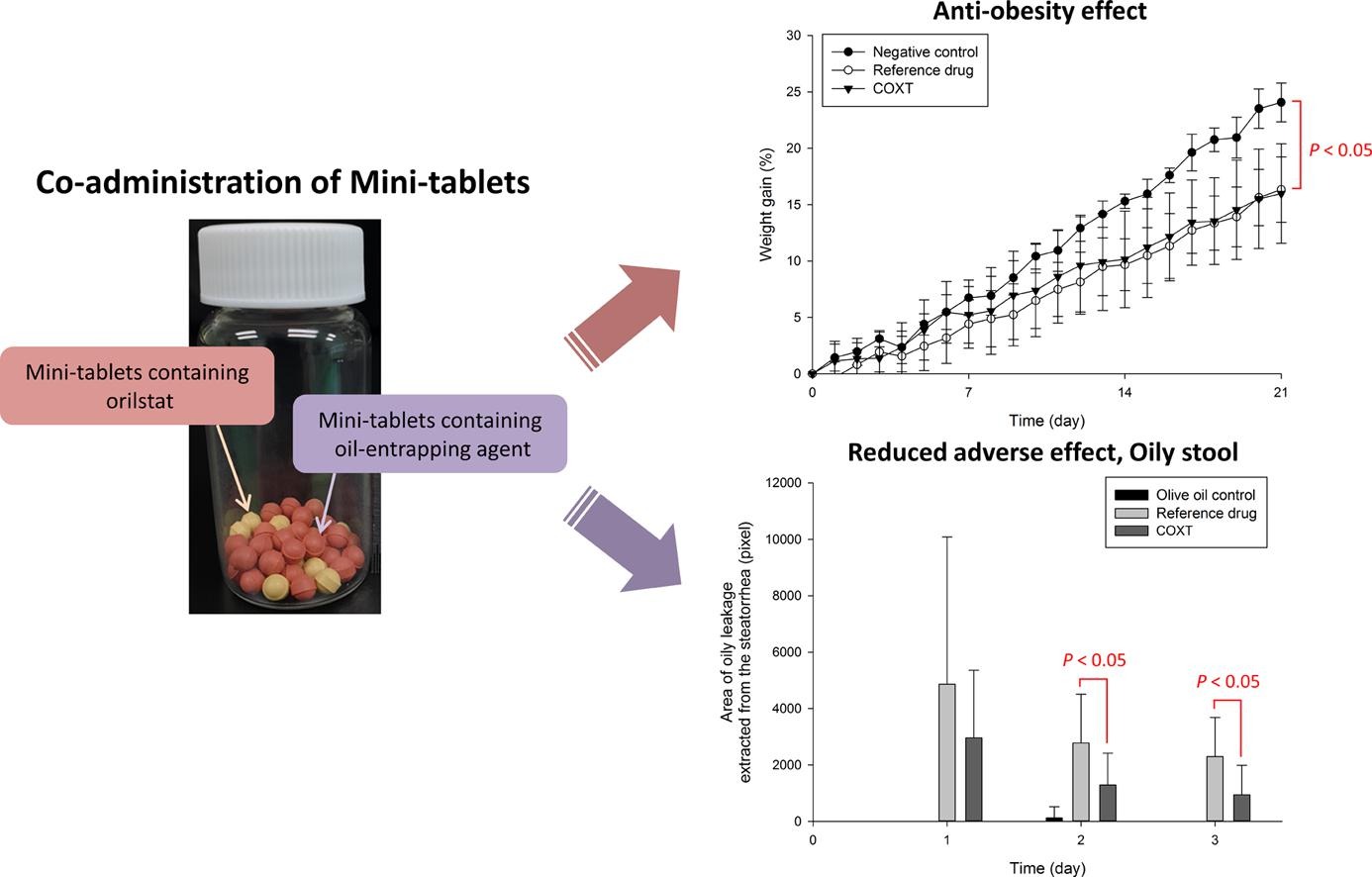
The purpose of this study was to develop an oral dosage form of orlistat for the treatment of obesity with reduced adverse effects, for example, fatty and oily stool that have been reported to be associated with the mechanism of action of orlistat. Based on the in vitro results obtained in this study, xanthan gum was selected as an oil-entrapping agent. Thus, the co-administration of mini-tablets containing orlistat and mini-tablets containing xanthan gum was proposed as the optimized dosage form for orlistat. The prepared mini-tablets showed an equivalent drug release profile with a similarity factor value, f2, more than 50 to that of commercially marketed orlistat immediate-release capsules, Xenical® capsules. In addition, the optimized formulation also showed the in vivo anti-obesity effects similar to those of Xenical® capsules. In particular, the analysis of feces excreted by Sprague-Dawley rats revealed that the optimized formulation resulted in significantly less oily stool, steatorrhea, than Xenical® capsules (P < 0.05).
Consequently, the proposed formulation, the co-administration of mini-tablets containing orlistat and mini-tablets containing xanthan gum, may be considered as a promising anti-obesity treatment with reduced adverse effects related to orlistat. Continue on Anti-obesity effect with reduced adverse effect of the co-administration of mini-tablets containing orlistat and mini-tablets containing xanthan gum
Keywords and Materials: Orlistat, Oily stool, Oily leakage, Xanthan gum, Anti-obesity, Reduced adverse effect, gum arabic, hydroxypropyl methylcellulose (HPMC, Metolose® 90SH-100,000SR), polyethylene oxide (PEO, Polyox™ WSR coagulant, sodium carboxylmethyl cellulose, microcrystalline cellulose (MCC-101, Ceolus™ PH-101 or MCC-102 Ceolus™ PH-102), hydroxyethyl cellulose (HEC, Natrosol™ 250HHX pharm), polyacrylic acid (PA, Carbopol® 974P), chitosan, colloidal silicon dioxide (SO, Aerosil® 200), magnesium aluminium silicate (MAS, Neusilin® US2), glyceryl behenate (GB, Compritol® 888 ATO), glycerol monooleate (GMO, Peceol™), polyoxyl 40 hydrogenated castor oil (CO, Kolliphor® RH 40), caprylocaproyl macrogolglyceride (CMG, Labrasol®), sodium starch glycolate (SSG, Primojel®), crospovidone (CP, Kollidon® CL), sodium lauryl sulfate, polyethylene glycol 400 ( d-α-tocopherol acetate (Vitamin E acetate,, polyvinylpyrrolidone (PVPK30, Kollidon® 30), magnesium stearate, potato starch

