Formulation and quality consideration of cannabidiol printed forms produced by fused-deposition modeling
Introduction
Cannabidiol (CBD) is a promising non-psychoactive cannabinoid that shows several clinical outcomes. Commercially, this molecule is used in Epidiolex® (Greenwich Biosciences, Inc), a Food and Drug Administration (FDA)-approved drug to treat Lennox-Gastaut and Dravet syndromes, which are childhood-onset epilepsy [1]. These two syndromes are difficult to treat and the dosage of Epidiolex® can reach a maximum of 10 mg/kg twice a day [2]. In addition to these indications, CBD has been studied for the treatment of different health problems, such as opioids use disorder, social anxiety, schizophrenia or cancers with a wide range of dosages varying from less than 1 mg/kg/day to 50 mg/kg/day [[3], [4], [5]]. For example, Hurd et al. recently performed a clinical trial to assess the efficacy of CBD to inhibit the drug cue-induced craving and anxiety in drug-abstinent individuals with heroin use disorder [6]. Moreover, some authors conducted a systematic review of clinical studies performed on CBD [7]. It first appeared that the tested CBD formulations were mainly administrated in the form of an oily solution, formulated in capsule or not. However, lipid formulations suffer from some drawbacks such as the sensitivity to oxidation or the compatibility with the capsule when encapsulation is considered [8]. The development of an oral solid dosage form of CBD would therefore be interesting for future investigations on its therapeutic effects. Secondly, among the trials that demonstrated positive effects of CBD on the treatment studied, the authors observed that the dosage of CBD can induce the variation of its therapeutic effect. Although clinical trials need to be conducted on a larger number of subjects, this suggests that in the future, clinicians will need to be able to formulate CBD drugs with adjustable dosages in order to be able to treat patients in the most effective way. In addition to these variable dosage challenges, the low bioavailability of CBD must be considered. Indeed, CBD is part of class II of the Biopharmaceutical Classification System (BCS). This drug shows poor aqueous solubility (0.01 μg/mL) and a high hepatic first pass metabolism which result in an estimated bioavailability of 6% [9]. Moreover, CBD absorption is erratic, leading to high variation of plasmatic concentrations in clinical population [[10], [11], [12]]. It is therefore crucial to develop strategies that improve CBD oral bioavailability with solid oral formulations which are preferred by patients and more stable than the liquid formulations.
Several techniques are used to improve the solubility of BCS II molecules, such as dissolution of the drug in lipid media, complexation into cyclodextrins, the impregnation of mesoporous silica or the formation of amorphous solid dispersions (ASDs), especially by hot-melt extrusion (HME) and spray drying [[13], [14], [15]]. Compared to the spray drying technique, HME has the advantage of working without the use of expensive and polluting solvents.
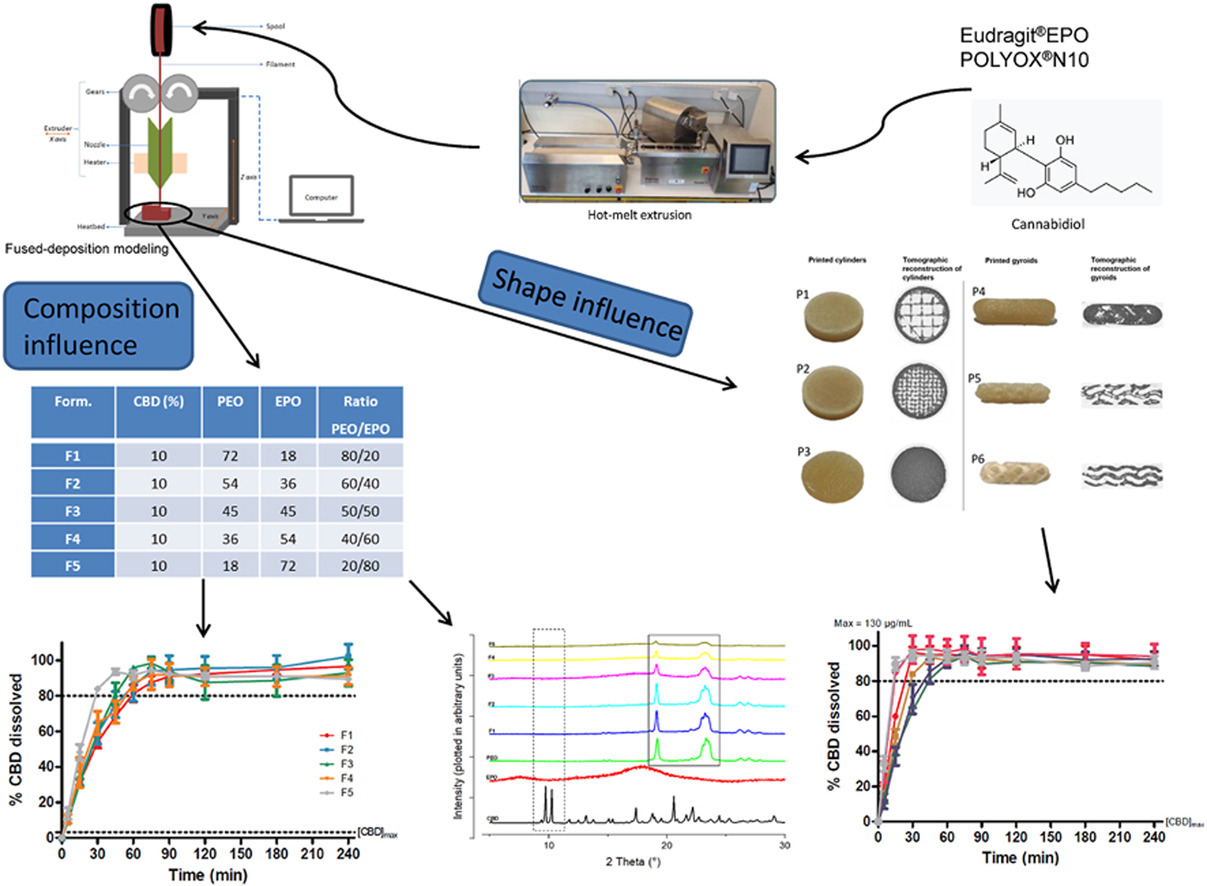
In a previous study, twin screw HME has already been used to increase the apparent aqueous solubility of CBD [16]. This technique involves the heating of a mixture between an active pharmaceutical ingredient (API) and one or more polymers along two screws, inside a barrel, until obtaining a filament in the shape of a spaghetti or a film, depending on the shape of the die. The filament can further, among other processes, be pelletized and filled into capsules, used directly as implant, be milled and compressed or shaped by 3D printing [[17], [18], [19], [20]].
Since the FDA-approval of the first 3D printed dosage form Spritam® manufactured using the drop-on-powder deposition (Aprecia Pharmaceuticals), the application of 3D printing has been extensively studied in the pharmaceutical field. 3D printing represents a great potential in drug formulation due to its flexibility, its ability to produce very complex structures, amorphous forms, dosage forms with multiple drugs without drug-drug incompatibility issues and on-demand manufacturing [21]. In addition, 3D printing makes it possible to move away from the one-size-fits-all manufacturing approach applied by conventional manufacturing techniques. Due to differences in genetics, weight, height, metabolism, health condition, age and tendency for compliance from patient to patient, there is a need for drugs with adaptable drug combinations, dosages and release rates to treat each individual adequately [20,22]. Therefore, the faculty of 3D printing to convert a great number of digital patterns into solid drug dosage forms with different dose or drug release makes 3D printing a great tool in personalized medicine. In this regard, fused deposition modeling (FDM) has already offered solutions to many challenges. This technique consists of driving a filament by two wheels into a print head in which it is melted. The molten filament is deposited, layer by layer, on a platform until obtaining a 3D object, previously designed on a computer [23]. FDM has been reported for the print multiple layered tablets, each layer with a different drug to reduce the risk of a missing dose by the patient [24]. In another study, attractive shapes were printed by FDM to increase the compliance of young patients [25]. Another example is the use of FDM to manufacture pulsatile delivery system to treat patients suffering from conditions that require complex release profile [26]. Moreover, it’s a low cost and simple method and it does not imply the use of organic solvents. These advantages could one day widespread decentralized drug manufacture by FDM, such as in hospitals or public pharmacies in which its advantages regarding the personalized medicine could be fully exploited. However despite the myriad of studies on the benefits of the 3D printing technique, there are still no guidelines regarding the evaluation of the performances or the quality control of the final 3D printed product [27]. Currently, the FDA recommends following the same production and control guidelines as conventional manufacturing techniques [28].
Therefore, this work aims to explore the development of a solid oral form of CBD with an immediate release printed by FDM. We have focused our research on the influence of the composition and the morphology of the formulation on the reproducibility of the process as well as on its pharmaco-technical performances based on the European Pharmacopoeia uncoated tablets monograph.
This study is divided into three parts. First, different combinations of two polymers mixed with CBD were extruded and printed in order to select the most suitable for the production of CBD printed forms with an immediate release (IR). According to the European Pharmacopoeia, an IR is achieved if at least 80% of the drug is released within 45 min (European Pharmacopoeia, Edition 11.0, monograph 5.17.1). This type of formulation should increase the CBD bioavailability. Indeed, Izgelov claimed that, due to its lipid nature, absorption of CBD mainly occurs in the upper part of the intestine, explaining the advantage of the dissolution of the drug in the stomach [29]. In this regard, IR formulation were chosen as they should dissolve early in the gastrointestinal tract, making dissolved CBD available to be absorbed early in the intestine. Secondly, the selected formulation was extruded and printed in six different designs and the influence of the morphology was evaluated in terms of reproducibility and quality. Finally, the dosage adaptability allowed by the FDM was studied. In this regard, three different dosages of CBD were characterized and compared.
Materials
CBD was purchased from THC Pharm (Frankfurt, Germany). Eudragit®EPO (EPO, amino alkyl methacrylate copolymer, Tg: 50 °C, soluble in water at pH < 5, gifted by Evonik, Germany) was used as matrix former. Poly ethylene oxide (PEO, POLYOX® WSR N10, MW 100000, gifted by Colorcon, UK), a semi-crystalline polymer (Tg = −67 °C and Tm = 65–70 °C) was used for its plasticizing effect.
Read more
Olivier Jennotte, Nathan Koch, Anna Lechanteur, Brigitte Evrard, Formulation and quality consideration of cannabidiol printed forms produced by fused-deposition modeling, Journal of Drug Delivery Science and Technology, 2023, 104837, ISSN 1773-2247,
https://doi.org/10.1016/j.jddst.2023.104837.

