Formulation and evaluation of gastric-floating controlled release tablets of Ginkgolides
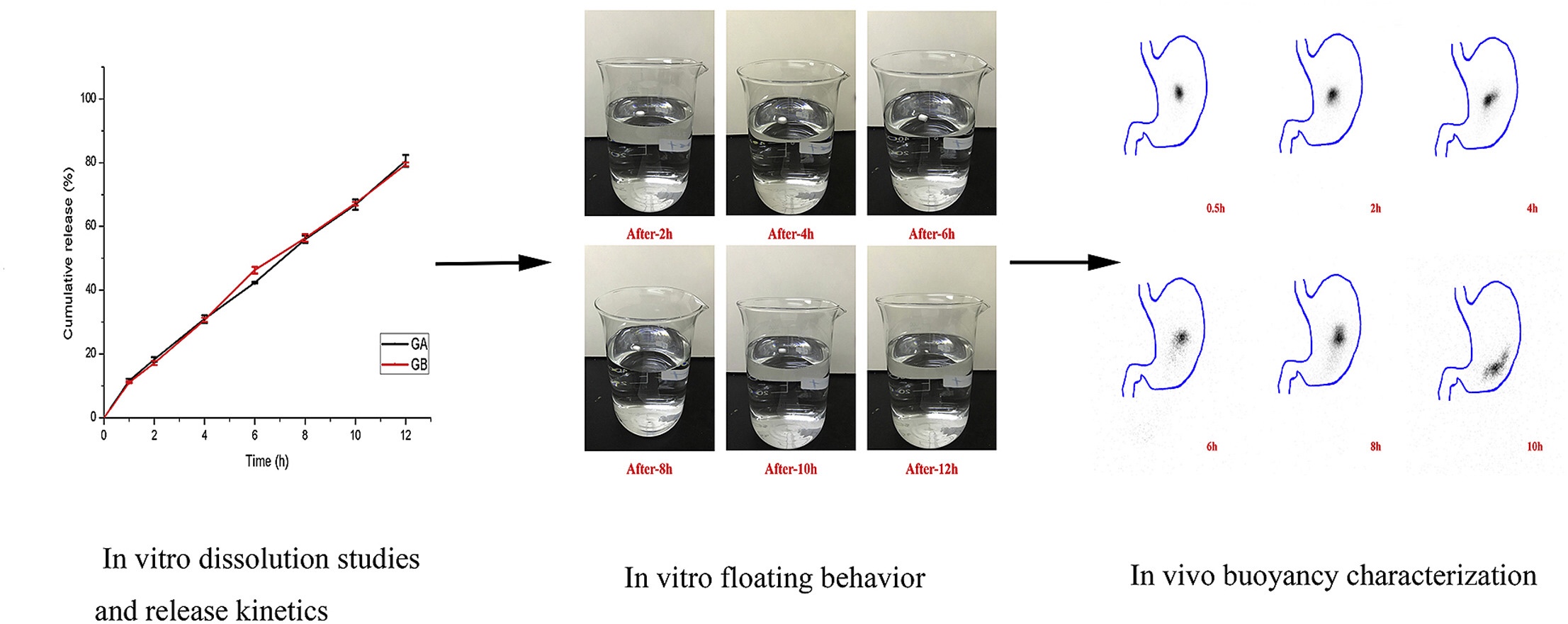
The present investigation was conducted to formulate and evaluate gastric-floating controlled release tablets of Ginkgolides. The target tablets were formulated by powder direct compression method combined with hydrophilic polymer, floating assistant agent, and effervescent substance. Formulations were evaluated for in vitro drug release, in vitro floating ability, and in vivo gastro-retentive behavior by gamma scintigraphy technique.
The optimized formulations showed good instant and total duration floating properties, and extended drug release characteristic for 12 h. The release behaviors of the tablets were fitted to zero-order model in the coupled action of drug diffusion and matrix erosion mechanism. In vivo behaviors of the tablets were observed at different time intervals from the radiographic pictures of the healthy volunteers and the retention time in the stomach was about 8 h. Results indicated that gastric-floating tablets of the Ginkgolides had the potential for a good gastric residence time and the controlled drug release. Continue on gastric-floating tablets

