The Future of Drug Delivery
Drug delivery technologies have been proven to improve treatment outcomes in many ways, including enhancing therapeutic efficacy, reducing toxicity, increasing patient compliance, and enabling entirely new medical treatments. As the therapeutic landscape has evolved from small-molecule drugs to a new generation of therapeutics including proteins, peptides, monoclonal antibodies, nucleic acids, and even live cells, drug delivery technologies have also evolved to meet their unique delivery needs. Starting from the advent of Spansule® sustained release technology in 1952, (1) a large amount of effort has been focused on extending the release of small-molecule-based drugs by entrapping them in dissolution-based formulations consisting of cellulose derivatives and cross-linked poly(acrylic acid) polymers; in biodegradable injectables based on poly(lactic-co-glycolic acid) (PLGA) and poly(lactic acid) (PLA); or in nondegradable implants based on poly(ethylenevinyl acetate), silicones, polyethylene, and other polymers. (2)
These approaches have enabled the long-term delivery of small-molecule therapeutics over a period from several days to weeks or months, thereby reducing the dosing frequency and improving patient adherence and compliance. Such long-acting delivery systems have revolutionized the treatment and management of multiple conditions, including psychotic disorders, pain, and advanced prostate cancer. (3−5) Recent FDA approval of Apretude®─a long-acting injectable of cabotegravir, an antiretroviral─has also demonstrated the enormous potential of long-acting approaches for pre-exposure prophylaxis (PrEP) of HIV and other infectious diseases. (6) The use of long-acting systems for localized drug delivery has also been shown to improve the therapeutic outcome by changing the local pharmacokinetics (PK) of the drug. Zilretta®, a PLGA microsphere-based intra-articular formulation of a corticosteroid, resulted in significantly higher drug levels in the knee joint compared to the free drug during the first few weeks postinjection, which translated to improved therapeutic benefit. (7) In parallel, nanoparticles have also emerged as an effective method of altering the biodistribution of small-molecule drugs, prolonging the half-life in systemic circulation, and enhancing tissue targeting.
Although a major focus for nanoparticles has been to achieve tumor-targeted delivery, the approved products Doxil® and Abraxane® were primarily focused on reducing side effects and extending circulation time rather than the superior efficacy of the drugs. (8) Drug delivery innovations over the past several decades also feature ambitious efforts to deliver macromolecules or biologics. Conjugating polyethylene glycol (PEG) to proteins increased their circulation time; about 20 PEGylated protein formulations have been developed to date. (9) In 2018, siRNA was successfully delivered to the liver using lipid nanoparticles (Onpattro®). (10) Subsequently, nanoparticle delivery technology laid the foundation for all-mRNA vaccines, which addressed the global COVID-19 pandemic. (11) Looking back, seven decades of effort have produced stunning drug delivery technologies to break down the barriers between new drug candidates and their targets in tissues and cells. Looking forward, the future of drug delivery is bright. The global pharmaceutical drug delivery market is projected to reach $2206.5 billion by 2026, exhibiting a compound annual growth rate (CAGR) of 5.9%. (12)
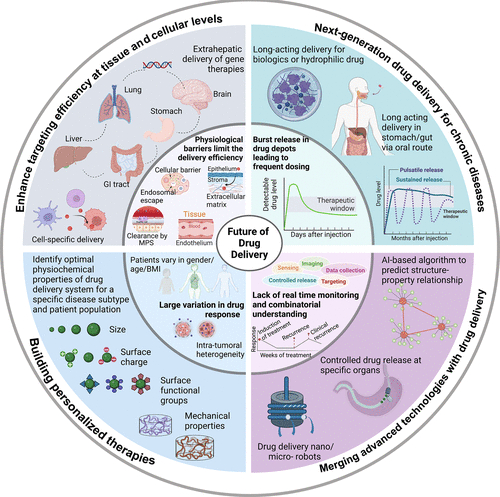
While the overall goal for drug delivery technologies remains transporting and maintaining therapeutic concentrations of a drug at the target biological site, we envision that the future will focus on:
(i) enhancing targeting efficiency at both the tissue and cellular levels and increasing the delivery of molecules through barriers such as the brain, intestine, skin, lung, and vagina;
(ii) developing next-generation long-acting delivery technologies to improve drug PK and achieve preprogrammed pulsatile release over a period of time;
(iii) developing drug delivery technologies for personalized therapies; and
(iv) merging advanced technologies, including machine learning, artificial intelligence
(AI), wireless, and soft electronics, with drug delivery. Success in these directions will require concerted efforts from broad interdisciplinary groups of scientists and researchers and strong backing from funding agencies, investors, hospitals, clinical trial groups, and other key stakeholders.
Download the full article as PDF here The Future of Drug Delivery
or read it here
Jingjing Gao, Jeffrey M Karp, Robert Langer, and Nitin Joshi, The Future of Drug Delivery, Chem. Mater. 2023, 35, 2, 359–363, Publication Date:January 24, 2023, https://doi.org/10.1021/acs.chemmater.2c03003

