Sustained release polymer and surfactant based solid dispersion of andrographolide exhibited improved solubility, dissolution, pharmacokinetics, and pharmacological activity
Andrographolide (AD) is a potent natural product with a wide range of pharmacological activities. However, it has low oral bioavailability due to poor solubility and dissolution rate. Solid dispersion (SD) is a promising technique to improve the solubility and dissolution rate of such molecules. In this study, SD formulation of AD was prepared using Kollidon-SR (KSR) and Poloxamer-407 (P-407) as carriers. SD was prepared using the solvent evaporation method and evaluated for the modulation of solubility of AD. The developed SD formulation was characterized using scanning electron microscopy (SEM), differential scanning calorimetry (DSC), powder X-ray diffraction (PXRD), and Fourier transform infrared spectroscopy (FTIR).
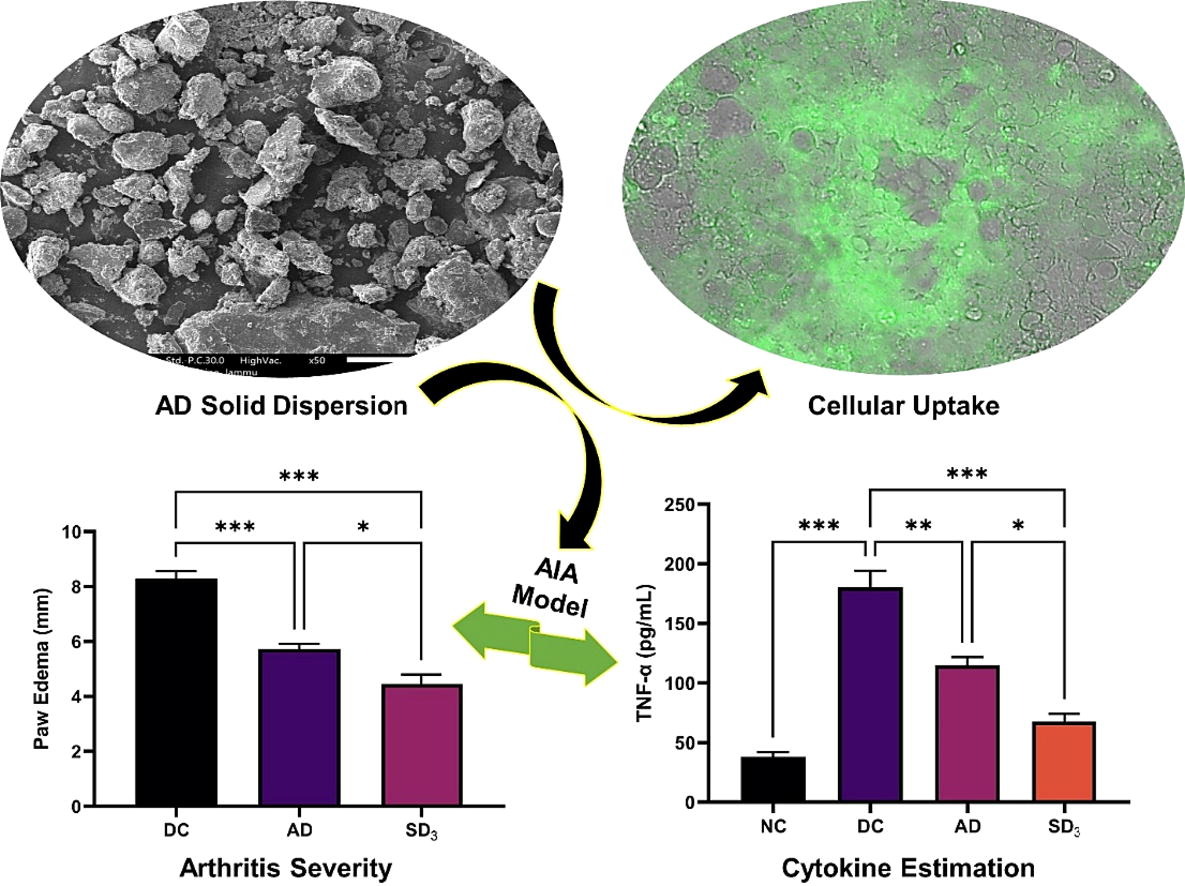
Further, dissolution rate, yield, drug content, stability, flowability, and pharmacokinetic profile of SD were evaluated. The compatibility of SD with the Caco-2 cells and its impact on the P-glycoprotein (P-gp) mediated efflux was also investigated. Furthermore, carrageenan-induced paw edema, and adjuvant-induced arthritic model were used to evaluate the efficacy of SD. The results showed that SD3 (AD+KSR+P-407, 1:6:8) exhibited the highest solubility and dissolution rate, and significantly improved pharmacokinetic profile compared to native AD. SD3 was found to be stable during storage and displayed excellent yield, drug content, and flowability.
This formulation was found to be compatible with the Caco-2 cells and retarded the efflux of P-gp substrate. SD3 also demonstrated substantially better efficacy than native AD in terms of paw edema inhibition (carrageenan-induced paw edema, Wistar rats), and overall improvement of disease condition (in terms of paw edema, arthritic score, AST, ALT, cytokines, radiological changes, and histopathology) in arthritic Wistar rats. In conclusion, SD3 exhibited improved solubility, dissolution rate, pharmacokinetic profile, and pharmacological activity than native AD.
Chemicals and reagents
AD (purity: ≥98.0%) from Tokyo Chemical Industry (Tokyo, Japan). Kollidon-SR, Poloxamer-407, MTT dye, Trypsin-EDTA, HBSS (Hanks’s balanced salt solution), and DMSO (Dimethyl sulfoxide) were procured from Sigma-Aldrich, India. EMEM with non-essential amino acid and Dulbecco’s Phosphate Buffered Saline (PBS) were purchased from Hi-Media. Fetal bovine serum (FBS), Penicillin-streptomycin, ELISA kits were from Invitrogen and other chemicals used were of research grade.
Read more
Syed Assim Haq, Gourav Paudwal, Nagma Banjare, Nusrit Iqbal Andrabi, Priya Wazir, Utpal Nandi, Zabeer Ahmed, Prem N. Gupta, Sustained release polymer and surfactant based solid dispersion of andrographolide exhibited improved solubility, dissolution, pharmacokinetics, and pharmacological activity, International Journal of Pharmaceutics, 2024, 123786, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2024.123786.

