Amorphous Solid Dispersions (ASDs): The Influence of Material Properties, Manufacturing Processes and Analytical Technologies in Drug Product Development
Abstract
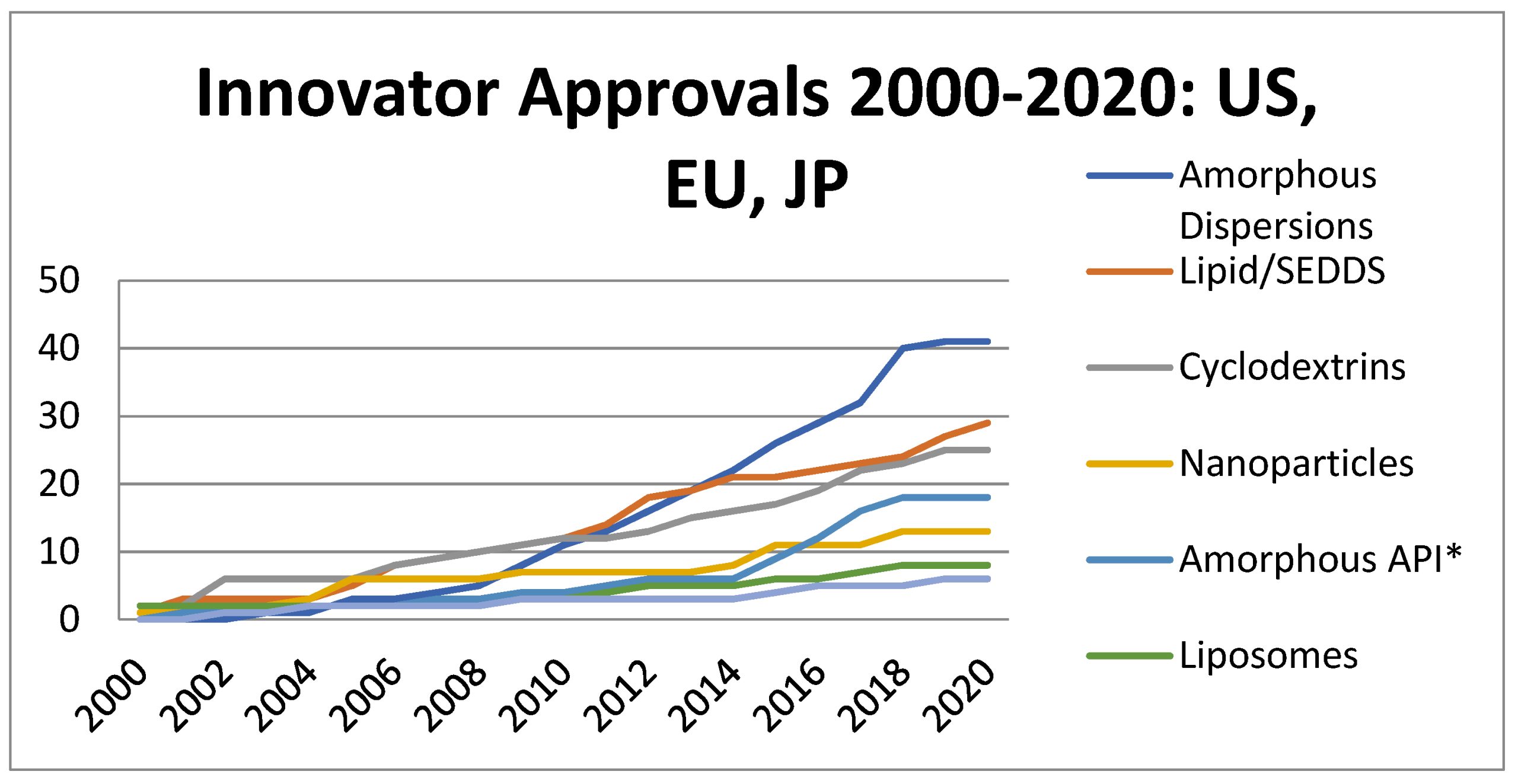
Introduction
The Noyes–Whitney equation [12] relates mass transfer to the concentration gradient as
where D is the diffusion coefficient (cm2/s), A is the cross-sectional area, h is the thickness of the hydrodynamic diffusion layer and Cs is the solubility or maximum concentration. Under infinite dilution (sink), the concentration gradient approximates to solubility Cs, resulting in
| Drug (Marketed Product) | Technology (1) | Polymer (2) | Dissolution Approach (3) FDA Recommended Method ( #9884; ) vs. Bio-Relevant Approach (∞) | Supporting Analytical Techniques | Conclusion |
|---|---|---|---|---|---|
| Reference product of Verapamil: ISOPTIN-SRE, ER tablets Developed formulation (tablets) [93] | HME (ISOPTIN-SRE) Kneading, solvent and co-precipitation method | HPMC/HPC 12 SDs prepared: 1:1, 1:2 and 1:3 API—polymer ratios with the following polymers: PVPK30, β-cyclodextrin, PEG 6000, HPMCK100M | #9884; Two-phase dissolution: phase 1: 900 mL SGF without enzyme 60 min; phase 2: withdraw and transfer to 900 mL SIF without enzyme, 7 h 50 rpm, USP II with wire helix ∞Phosphate buffer pH 1.2, 900 mL, 50 rpm, USP I | DSC, PXRD, SEM, FTIR, supersaturation solubility testing, stability studies | Increased dissolution rates of tablets containing SD with API: PEG6000 ratio of 1:3 in comparison to other formulations and marketed tablets due to decreased particle size, increased wettability and dispersibility of verapamil; Drug–carrier interaction observed; Higher polymer concentration gives faster drug release. |
| Reference product of Itraconazole: Sporanox cps, and ONMEL tbl Developed formulation (SD, tablets) [94] | Spray lavering (Sporanox) HME (ONMEL) Solubilization in concentrated aqueous solutions of weak organic acids and drying | HPMC SDs with 2–20% drug load prepared with Glutaric acid | #9884; 0.1 N HCl, 900 mL 75 rpm, USP II (tbl) SGF without enzyme, 900 mL, 100 rpm, USP II (cps) ∞0.1 N HCL, 250 mL 75 rpm, USP II (ASD) | DSC, PXRD, ATR-FTIR, pH-solubility studies | Solubility greatly enhanced compared to amorphous form of drug, possible weak drug–acid interactions observed; precipitated as mostly nanoparticles that enable rapid re-dissolution, which might influence absorption. |
| Reference product of Tacrolimus PROGRAF Developed formulation (SD) [95] | Spray drying/fluid bed (PROGRAF) Spray drying via solvent-evaporation method, solvent-wetting method, or surface-attached method – three different processing methodologies | HPMC 3SDs prepared: 10:80:1 API:HP-β-CD:DOSS ratio | #9884; 0.005% HPC in Water with 0.50% SLS adjusted to pH 4.5, 900 mL, 100 rpm, USP II (tbl) #9884; HPC solution (1 in 20,000), adjusted to pH 4.5 by phosphoric acid, 900 mL 50 rpm, USP II (cps) ∞0.005% HPC in Water with 0.50% SLS adjusted to pH 4.5 by phosphoric acid, 500 mL 50 rpm, USP II with sinker (SD) | SEM, DSC, PXRD | The solubility and dissolution were significantly improved by SD preparation method compared to drug powder. |
| Reference product of Nifedipine Afeditab Developed Tablets [96] | Melt/absorb on carrier Co-precipitation | Poloxamer or PVP 12 SDs prepared:1:1, 1:5 and 1:10 API:polymer ratio with all listed polymers: poloxamer, HPMC, PEG 4000 and PEG 6000 [36]; with each API-Polymer ratios of 1:1, 1:5 and 1:10 tested | #9884; 0.5% SLS in SGF without enzyme pH 1.2, 900 mL 100 rpm, USP II (tbl ER) #9884; SGF without enzyme, 900 mL 50 rpm, USP II (cps) ∞SGF without enzyme, 900 mL 50 rpm, USP XXI (SD, tbl) | DSC, FT-IR | SD tablets prepared with PEG 6000 and poloxamer showed better release profile than marketed products. |
| Reference product of Griseofulvin (Gris-PEG) Developed formulation (SD) [97] | HME (Gris-PEG) Solvent evaporation technique | PEG 6000 24 SDs prepared: 3:1, 1:1, 1:2 and 1:9 API:polymer ratios with all polymers: PVP, HPMC, and Eudragit L 100, Eudragit E 100, Eudragit S 100, PEG 8000 | #9884; 4.0% SLS in water, 1000 mL 75 rpm, USP II (tbl) #9884; 0.54% SLS in water, 1000 mL 25 an 50 rpm, USP II (susp) ∞Dissolution studies not performed. | PXRD, mDSC, ATR-IR, Raman spectroscopy | Increased polymer concentration leads to lower drug released because drug binds tighter to the concentrated polymers, however SD is more stable. |
| Reference product of Nimodipine Nimotop Developed formulation (SD) [98] | Spray drying (Nimotop) HME | PEG 9 SDs prepared: 1:2, 3:7 and 1:9 API:polymer ratio with polymers: HPMC, PVP-VA, Eudragit EPO | #9884; 0.5% SDS in water, 900 mL 50 rpm, USP II (cps) ∞0.05% SLS in acetate buffer pH 4.5, 900 mL 75 rpm, ZRS-8G (paddle) | DSC, XRPD, FT-IR, SEM | Eudragit EPO and PVP-VA showed better miscibility than HPMC. Drug–polymer hydrogen bonding was observed. |
| Reference formulation of Lopinavir & Ritonavir KALETRA tablets and capsules Developed formulation (SD) [99] | HME (KALETRA) Solvent granulation process | PVP-VA SDs with various API1:API2:PVP-VA ratios | #9884; Tier 1:0.06 M polyoxyethyelene 10 lauryl ether with 10 mM sodium phosphate monobasic (pH 6.8) #9884; Tier 2: same as tier 1 with no more than 1750 USP units/L of pancreatin, 900 mL (cps) 50 rpm, USP II #9884; Test 1: 0.06 M decaethyelene glycolmonododecyl ether in water #9884; Test 2: 37.7 g/L of polyoxyethyelene 10-lauryl ether in water (tbl) 75 rpm, USP II ∞10 mM phosphate buffer pH 6.8, 250 mL and 0.1 N HCl, 250 mL 150 rpm, jacketed beaker | XRPD, FT-IR | Molecular mixing of both components into a single amorphous phase negatively impacts ritonavir dissolution performance in comparison with marketed formulation. Amorphous suppression phenomenon observed in pH-shift dissolution method. It is proposed that dissolution of ritonavir from the surface of the particles in acidic media leaves behind a lopinavir-rich surface which acts as a barrier for the remaining ritonavir to dissolve. |
| Reference product of Fenofibrate Fenoglide Developed formulation (SD) [100] | HME (Fenoglide) Solvent evaporation method | PEG/Poloxamer 188 7 SDs prepared: 1:1, 1:2 and 1:3 API:polymer ratio with polymers: Carplex 80 and PEG 4000 and 1:5:6 API:polymer ratio with Carplex 80 and PEG 6000 respectively | #9884; 25 mM/50 mM/0.75% SLS in water, 1000/1000/900 mL (40 and 120 mg/48 and 145 mg/54 and 160 mg tbl) 50/50/75 rpm, USP II #9884; Phosphate buffer w/2% Tween 80 and 0.1% pancreatin pH 6.8, 900 mL 75 rpm, USP II (cps) ∞Demineralized water, 900 mL 50 rpm, USP II | DSC, PXRD, FT-IR, SEM | The most significant improvement of drug dissolution and amorphization was obtained with SD prepared with drug:Carplex:PEG ratio 1:5:6 |
| Reference product of Ivacaftor KALYDECO Developed formulation (SD) [101] | Spray drying (KALYDECO) HME | HPMCAS 9 SDs prepared: 1:1 API: polymer (Soluplus, HPMC, Copovidone), each pair with three surfactants (SLS, poloxamer, polysorbate 70) | #9884; 50 mM sodium phosphate buffer with 0.7% SLS pH 6.8, 900 mL (tbl) 65 rpm, USP II with a sinker ∞50 mM sodium phosphate buffer pH 6.8, 900 mL 65 rpm, USP II | XRPD, DSC, FT-IR | Improved solubilization by improved wetting of drug substance by hydrophilic carriers which represent rich microenvironment formed at the surface of the drug substance and this leads to improved dissolution rate. No defined drug–polymer interaction was observed. |
| Reference product of Posaconazole Noxafil Developed formulation (SD, tablets) [102] | HME (Noxafil) Spray drying | HPMCAS 1 SD prepared: 3:1 API:polymer ratio with polymer Eudragit L100 | #9884; Acid Stage: 0.01 N HCl, 750 mL; Buffer Stage: 50 mM phosphate buffer, pH 6.8 with 0.37% Polysorbate 80 (after 120 min, to the acid stage, add 250 mL of 0.2 M Phosphate Buffer, 1.46% Polysorbate 80) (tbl DR) 75 rpm, USP II #9884; 0.3% SLS, 900 mL 25 rpm, USP II (susp) ∞0.01 M HCl with 34 mM NaCl solution and phosphate buffer with SIF powder pH 6.5 CTD apparatus | mDSC, PXRD, SEM, in-vivo study | The in-vitro dissolution data underpredicted in-vivo performance, potentially due to higher driving force for precipitation in-vitro versus in-vivo. Including a concentration-sustaining polymer extragranularly to SD but inside tablet was as effective as including it inside the ASD itself. |
| Reference product of Everolimus CERTICAN and ZORTRESS Developed formulation (SD, tablets) [103] | Melt or spray drying (CERTICAN, ZORTRESS) Solvent-wetting and co-precipitation methods | HPMC SDs with various API:polymer ratios with HPMC were prepared | #9884; Water with 0.4% sodium dodecylsulfate, 500 mL (tbl) 50 rpm, USP II ∞0.4% SLS solution in water and distilled water, dissolution media pH 1.2, pH 4.0 and pH 6.8, 900 mL 50 rpm, USP II | XRPD, SEM, particle size analysis, stability and in-vivo studies | The optimized SD consisted of drug:HPMC weight ratio of 1:15. Tablets with SD created with solvent-wetting technique showed identical release rate to that of commercially available product. |
| Reference formulation of Telaprevir INCIVEK Developed formulation (SD) [104] | Spray drying (INCIVEK) Co-milling with polymers | HPMCAS 3 SDs prepared: 1:1 API:polymer ratio with polymers: PVP-K30, PEG 6000, HPMC | #9884; 1% SLS in water, 900 mL (tbl) 50 rpm, USP II ∞Distilled water, 0.1 M HCl pH 1.2, phosphate buffer pH 6.8, 900 mL 100 rpm, ZRC-8D (paddle) | XRPD, DSC, SEM, FT-IR, cytotoxicity evaluation, stability studies | Hydrogen bonding drug–polymer interaction observed. Drug–polymer SD did not affect efficacy of the drug and showed no toxic side effects to normal liver cells. No comparison to reference product shown. |
| Reference formulation of Vemurafenib ZELBORAF Developed formulation (SD, capsules) [19] | Co-precipitation method (ZELBORAF) Co-precipitation method | HPMCAS 3 SDs prepared: 2:3 API:polymer ratio with HPMCP, HPMCAS, and Eudragit L 100-55 | #9884; 1% Hexadecyltrimethylammonium bromide in 0.05 M phosphate buffer pH 6.8, 900 mL (tbl) 75 rpm, USP II ∞0.05% hexadecyltrimethylammonium bromide in phosphate buffer pH 6.8 10 mL/min, USP IV ∞FaSSIF, 900 mL 75 rpm, USP II [101] | XRPD, DSC, SEM, stability and in-vivo studies | Among used polymers, HPMCAS was found to be the best to prepare stable SD, based on superior physical stability and faster dissolution. No dissolution comparison to reference product shown. |
| Reference product of Ritonavir NORVIR HIV Developed formulation (SD) [105] | HME (NORVIR HIV) Solvent evaporation and melt method | PVP-VA 4 SDs prepared: 1:4 API:polymer ratio polymers Gelucire, sorbitol (with both listed method): | #9884; 60 mM polyoxyethyelene 10 lauryl ether, 900 mL (tbl) 75 rpm, USP II #9884; 0.1 M HCl with 25 mM polyoxyethyelene 10-lauryl ether (cps) 50 rpm, USP II ∞0.1 M HCl, 900 mL FaSSIF pH 6.5, 500 mL ∞FeSSIF pH 5.0, 1000 mL 50 rpm, USP II with sinkers | DSC, XRPD, TEM, FT-IR, in-vivo study | Hydrogen bonding was observed in SD resulting in increased drug solubility as compared to pure drug. Maximum dissolution was obtained with FeSSIF media, which confirmed food-related absorption of drugs. No comparison to reference product available. |
Download the full study as PDF here Amorphous Solid Dispersions (ASDs): The Influence of Material Properties, Manufacturing Processes and Analytical Technologies in Drug Product Development
or read it here
Iyer, R.; Petrovska Jovanovska, V.; Berginc, K.; Jaklič, M.; Fabiani, F.; Harlacher, C.; Huzjak, T.; Sanchez-Felix, M.V. Amorphous Solid Dispersions (ASDs): The Influence of Material Properties, Manufacturing Processes and Analytical Technologies in Drug Product Development. Pharmaceutics 2021, 13, 1682. https://doi.org/10.3390/pharmaceutics13101682

