Applicability of Co processed Excipient to ODT prepared by Direct Compression Continuous Manufacturing
Introduction
Continuous Manufacturing (CM) is a manufacturing method in which raw materials or blended materials enter the manufacturing process continuously, and products are discharged continuously throughout the duration of the process1). CM can yield required quantities of products with desired quality in a required period by continuous process operation. With the advancement of formulation technology including equipment and process analytical technology, several pharmaceutical companies have started producing solid dosage forms by CM, additionally even CDMOs start introducing CM2). However, there are technical and regulatory challenges to be overcome to spread CM. One of the challenges is minimizing a lot-to-lot variation of raw materials.
In the general batch production, the impact of lot-to-lot variation on product quality can be precisely controlled with sophisticated technique optimizing operating conditions and process management based on accumulated experiences. On the other hand, CM, which is a relatively new technology, has insufficient data accumulation compared to the batch production. The lot-to-lot variation may not negligible and can easily lead to instability in product quality in continuous process.
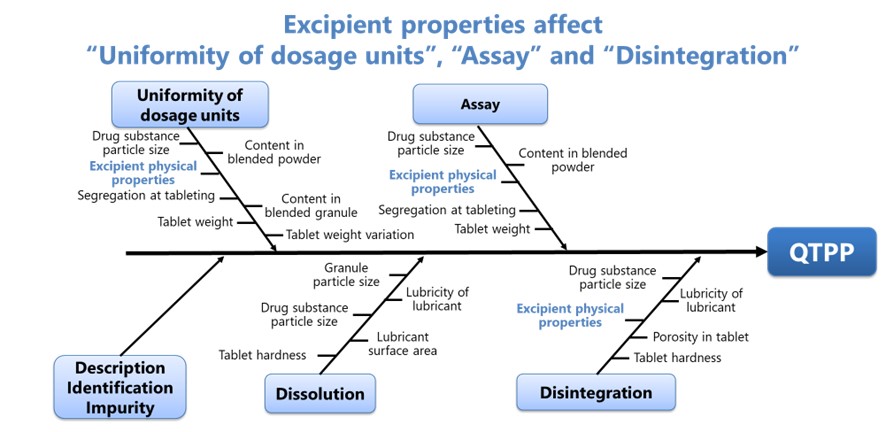
Fig.1 is an Ishikawa diagram for orally disintegrating tablets (ODTs) produced by CM, based on the diagram published by JPMA, and modified from the viewpoint of excipients manufacturers3). Here, the physical property of excipients is derived as one of the pre-critical material attributes (p-CMAs) which influences multiple critical quality attributes (CQAs) and ultimately also quality target product profile (QTPP). The property includes particle size, particle shape, flowability and compactability.
Especially for ODTs, water absorption property can be p-CMAs. When multiple raw materials are used for preparation of oral solid dosage forms by CM, there is concern that accumulation of small lot-to-lot variations in each raw material may bring critical defective impact on product quality. While co-processed excipients (CPEs) have possibility to eliminate the concern as they allow multiple excipients to be treated like a single excipient.
HiSORAD HSR-D03 (HSR) is one of the CPEs for direct compressible ODTs 4). In this study, ODTs with HSR were prepared by direct compression continuous manufacturing (DCCM), since HSR is designed to offset and homogenize the lot-to-lot variations in each raw material, contributing to the stabilization of product manufacturing and quality.
Test conditions and the QTPP
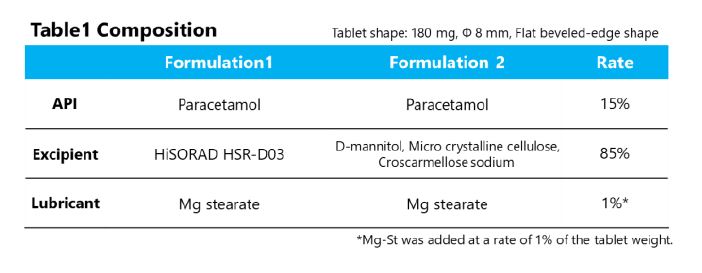
The tablet composition is shown in Table 1. HSR was applied to formulation 1. The comparison is the physical mixture (PM) of the same composition as HSR (Formulation 2).
The QTPPs were set: coefficient of variation (CV) of tablet weight <1.0%, tablet hardness >50 N, and disintegrating time < 30 sec.

Results and discussion
Tablet stability in DCCM
The CV of tablet weight against to operating time are shown in Fig. 2. Formulation 2 with the PM showed a large CV and did not achieve the QTPP. While in formulation 1 with HSR, the CV was less than 1% during the whole operating time, indicating passing the QTPP.
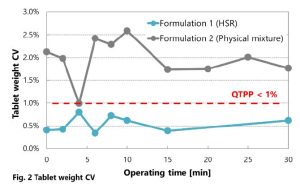
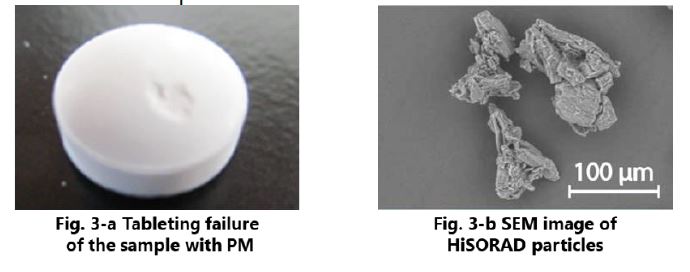
Although poor flowability of Paracetamol (AAP) deteriorates CV of tablet weight, good flowability of HSR contributes to improve whole powder flowability in formulation 1, resulting good weight uniformity. In addition, chipping was observed in formulation 2 only (Fig. 3-a). This is thought to be due to the fact that granulation in manufacturing process of HSR reduces amount of micronized D-mannitol, which often introduces tableting failures. Furthermore, the specific granulation for HSR increases inter-particle bonding force, which relatively weaken adhesion to the punches.
Content uniformity in DCCM
Fig. 4 and 5 show content rates of AAP at certain times. The content value in formulation 1 was closer to 100 % overall than that of formulation 2. Especially in the early stage of tableting, large variation was observed in formulation 2 (Fig. 5). The unique shape of HSR, which is non-spherical shown in Fig. 3-b, is thought to hold AAP particles so that good content uniformity was achieved 5).
Download the complete research paper as PDF here:
(just click the picture)

ODT performance
Next, tablet hardness and disintegration time were evaluated. Again, formulation 2 showed a large variation in tablet performance. Within 10 min of operating time, it was observed that some of samples could not achieve enough tablet hardness more than 50 N or disintegration time less than 30 s. Whereas, formulation 1 could meet the QTPP over the whole measured operating time (Fig. 6,7).
The primary particles are less likely to separate in formulation 1 since HSR is granulated, contributing homogeneity in ODT performance. However, in formulation 2, the segregation of the primary particles easily occurs, leading a non-uniformity in the mixture. As the result, variation of composition affects the quality profiles of tablet. For example, tablet hardness decreases in a tablet with less microcrystalline cellulose particles, and disintegration time is prolonged in a tablet with fewer croscarmellose sodium particles.
These results were confirmed that the co-processed excipient, HSR is able to suppress tableting failures in DCCM and stabilize the production of ODTs with practical CV of tablet weight and content uniformity. In future study, the impact of lot-to-lot variations in excipients on product quality will be evaluated.
Summary
The application of HSR enable to continuous manufacture ODTs with stable quality. Compared to the physical mixture, HSR resulted in more stable weight CV, less variation in content uniformity, better tablet performances. It was also effective in reducing tableting failures. These suggest that a co processed excipient can be applied in CM, and can contribute to maintain stable production and quality.
DAICEL CORPORATION
Sales and Marketing, Life Sciences Business Division
2 18 1 Konan, Minato ku, Tokyo, 108 8230, Japan
TEL: +81 3 6711 8222, FAX: +81 3 6711 8228
Contact: daicel [email protected]
Order a sample from Daicel or get more information:

