CombiLac® – MEGGLE’s co-processed lactose grades for direct compression

MEGGLE is a pioneer in co-processing technologies that allow simple, robust formulation development and manufacture. Through co-processing, MEGGLE developed highly functional excipients possessing unique qualities for directly compressible immediate and sustained release pharmaceutical solid dosage forms.
Read the news of MEGGLE´s product CombiLac® in the following article below and/ or download the technical brochure:
CombiLac®
General information
Direct compression (DC) tablet manufacture is a popular choice because it provides the least complex, most cost effective process to produce tablets compared to other tablet manufacturing approaches. Manufacturers can blend APIs with excipients and compress, making dosage forms simple to produce [1, 2].
DC technology and the use of modern tableting equipment require that excipients and APIs form a compactable mixture with excellent flowability and low particle segregation tendency [3].
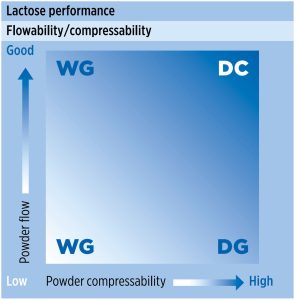
In the pharmaceutical industry, lactose is one of the most commonly used excipients; however, like many other excipients, lactose may not be suitable for direct compression without modification due to insufficient powder flow or/and compaction properties (figure 1).
Product description
The high-functionality excipient, CombiLac® is an integrated, lactose-based, co-processed excipient, specifically designed to ease oral solid dosage form development and manufacture. It is made up of 70 % alpha-lactose monohydrate, 20 % microcrystalline cellulose (MCC) and 10 % white, native corn starch, each conforming with Ph. Eur., USP-NF, and JP compendial requirements. The three individual components are integrated into a monoparticulate structure, which is not separable by physical means. CombiLac® shows improved compaction properties compared to an equivalent admixture of individual ingredients, providing robust tablets with minimal friability. It assures rapid,
hardness-independent tablet disintegration for effective API release, and features powder flow characteristics necessary to enhance dosage form weight uniformity and throughput in DC.
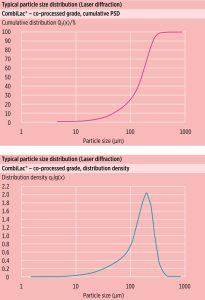
Particle size distribution (PSD)
Figure 2 depicts typical laser diffraction particle size distribution data for CombiLac®. The narrow PSD supports homogenous powder blend preparation, an important requirement in tableting manufacture.
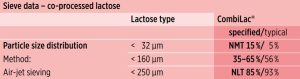
Figure 3 depicts the specified PSD range and typical average values by air-jet sieving. These parameters are constantly monitored through in-process control (IPC) testing and are part of the CombiLac® particle size distribution specification (Typical values shown for orientation only).
Core benefit
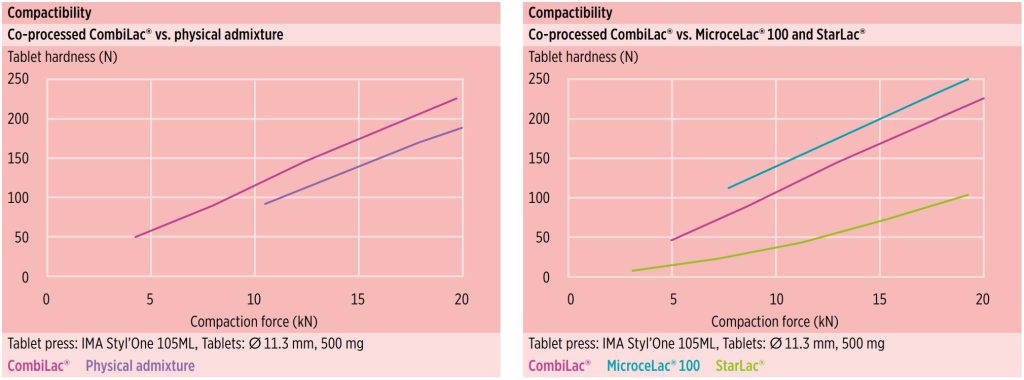
CombiLac® is highly appropriate for DC, as it synergistically combines the benefits of its individual components through intelligent particle design. The monoparticulate structure of CombiLac® clearly outperforms the physical blend in flow, hardness, and disintegration performance.
Powder compressibility
Material fill characteristics and compression behavior of formulation ingredients impact tablet quality. Generally, compaction performance is enhanced by combination of brittle and plastically deforming materials. However, addition of elastically deforming components, e. g. various starches, seems to be diametrically opposed. Pharmaceutical practice is often positioned to balance the integrity of a solid dosage form and its function as a pharmacological vehicle. CombiLac® is well-balanced by insuring sufficient tablet hardness and, simultaneously, fast disintegration time.
Additionally, CombiLac® offers superior hardness yield in comparison to the physical admixture of individual ingredients. An increase of approximately 20 % is achieved (figure 8).
Tablet hardness profiles of co-processed excipients MicroceLac® 100 (75 % alpha-lactose monohydrate and 25 % MCC) and StarLac® (85 % alpha-lactose, and 15 % native corn starch) are provided for reference (figure 9)
See the full brochure on “CombiLac®” here
(click the picture to download the brochure)
Benefits CombiLac®
- Excellent compactibility
- Excellent flowability
- Fast, hardness-independent tablet disintegration for effective API release
- Low friability
- Overcomes individual ingredient compaction and handling limitations
Source: MEGGLE technical brochure “CombiLac®”
See the overview video of the MEGGLE Dry Powder Inhalation product range here:
Company: MEGGLE is one of the world´s leading manufacturers of pharmaceutical grade lactose and co-processed excipients with expertise of more than 70 years. We encounter lactose in so many areas of our life – reason enough to take a closer look at this multi-functional “white powder”.
Do you need more information or a sample of CombiLac® excipients?


