Comparative Study of Isomalt and Co-processed Isomalt
ABSTRACT
Objective: The study aims to compare the tableting qualities of isomalt and co-processed isomalt.
Methodology: Co-processed isomalt prepared by melt granulation method evaluated for flow property, dilution potential, Kawakita plot, consolidation index, tabletability, Heckel plot and elastic recovery and compared with isomalt.
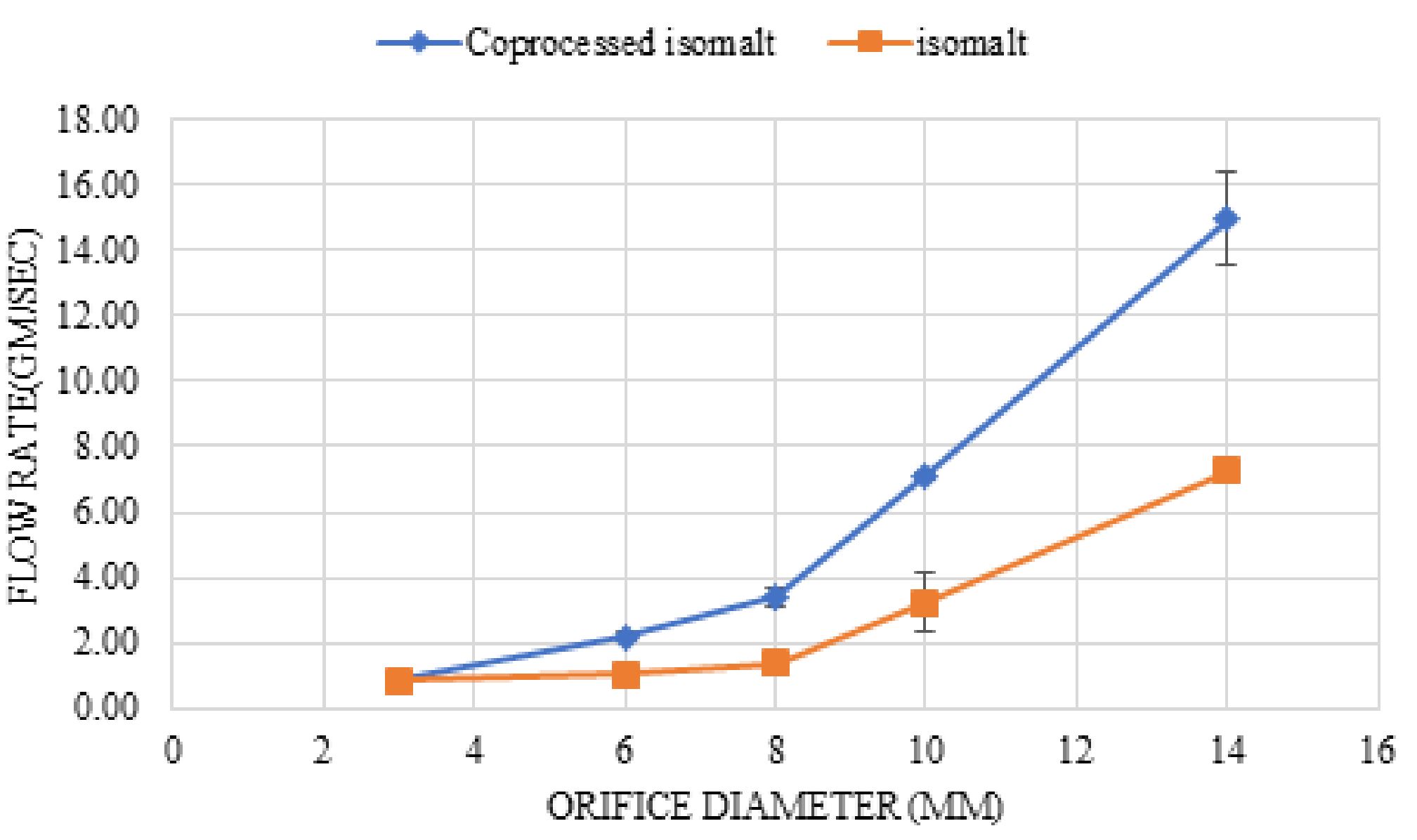
Result and Discussion: The co-processed isomalt demonstrated superior packing ability compared to isomalt and got
rearranged in the early compression stage, as per modified Kawakita equation. Co-processed isomalt had 40% dilution potential for paracetamol as compared with 20% for isomalt. Co-processed isomalt showed 40% dilution potential for ascorbic acid, and nimesulide, whereas mefenamic acid and aspirin showed 30% dilution potential. Co-processed isomalt overcomes the lamination and sticking problem of isomalt which has better tabletability.
INTRODUCTION
Direct compression is most suitable technique for manufacturing of tablets due its simplicity and cost-effectiveness. However, DC process is highly influenced by powder characteristics such as flowability and compressibility. Flowability and compressibility of powder is mainly depends upon the properties of drug and excipients.1 Scientists must now create excipients promptly with minimal expenses associated with manufacturing, scaling up, and the environment. However, seeking for new excipients necessitates expensive and time-consuming toxicological investigations. Because of this, only a small number of unique excipients have been released to the market in the past three decades, compared to new grades of existing excipients. An excipient is deemed novel if it contains an entirely novel chemical entity, is physically modified, is a co-processed mixture of current excipients, is directed toward a new route of administration. The advantage of co-processed material is improved flow properties (e.g. Fujicalin®2 and Cellactose), compressibility (Ludipress, Cellactose and SMCC), better dilution potential (Cellactose), and reduced lubricant sensitivity.3 The above functionality of the excipient are determined by fundamental parameters such as morphology, particle size, shape, surface area, porosity, and density. To improve functioning, user could alter the powder’s particle size and density.4
Directly compressible co-processed excipients have been available in the market include Ludipress, Cellactose, Microlac, StarLac, and Prosolv.5 A comparison of functional property of co-processed excipients like Cellactose6 and LubriTose SD7 with its physical mixture shows the co-processed excipient has better functionality than its physical mixture. Isomalt was co-processed by melt granulation method with PEG 4000 and crospovidone. The purpose of study is to check whether the flowability and tabletability of co-processed isomalt is better than isomalt by evaluating its flow property, dilution potential tabletability, heckle plot and elastic recovery.
Download the full article as PDF here Comparative Study of Isomalt and Co-processed Isomalt
Materials
Isomalt (GalenIQ 721, BENEO-Palatinit GmbH, Germany) was a generous gift sample from SPA Food and PharmaIngredients Pvt. Ltd., (Thane, Maharashtra, India). Aspirin was purchased from Modern Industries Nashik, ascorbic acid from Loba Chemie Pvt. Ltd. Mumbai, paracetamol was purchased from Research-Lab Fine Chem Industries (Mumbai, Maharashtra, India). All other laboratory chemicals were of analytical grade.
International Journal of Drug Delivery Technology (2023); DOI: 10.25258/ijddt.13.3.13, Sandhan SB, Wagh MP. Comparative Study of Isomalt and Co-processed Isomalt. International, Journal of Drug Delivery Technology. 2023;13(3):854-859
Read also the article about galenIQ™:


