Detachable-Dissolvable-Microneedle as a potent subunit vaccine delivery device that requires no cold-chain
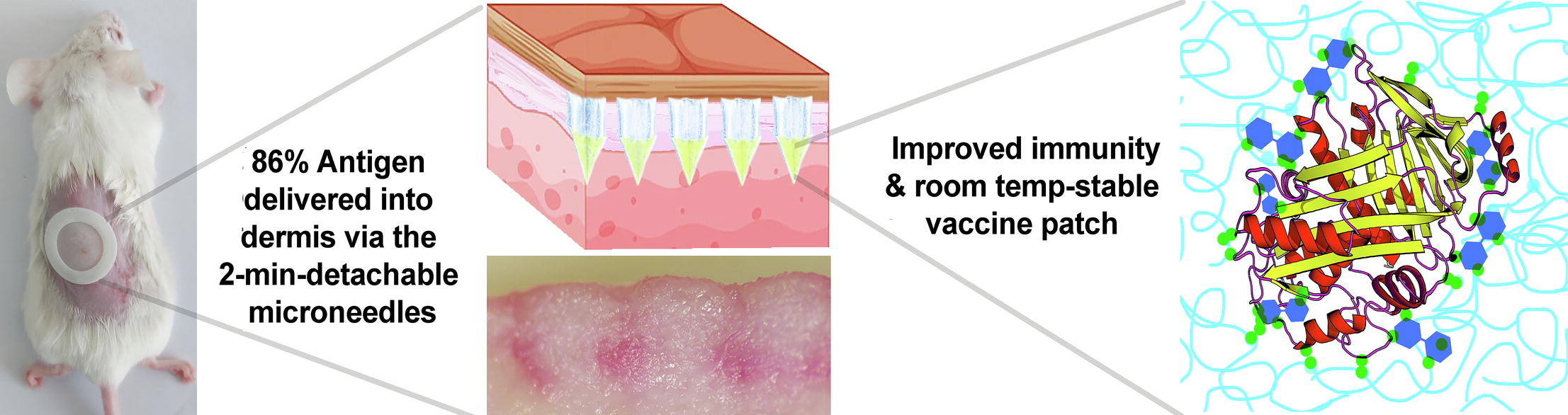
Although vaccine administration by microneedles has been demonstrated, delivery reliability issues have prevented their implementation. Through an ex vivo porcine skin experiment, we show visual evidence indicating that detachable dissolvable microneedles (DDMN) can deposit cargo into the dermis with insignificant loss of cargo to the stratum corneum. Using ovalbumin (OVA), a model antigen vaccine, as a cargo, the ex vivo experiments yielded a delivery efficiency of 86.08 ± 4.16%. At room temperature, OVA could be stabilized for up to 35 days in DDMN made from hyaluronic acid and trehalose.
Highlights
- Drug at tip detachable microneedles (DDMN) are developed for transdermal immunization of subunit vaccine.
Drug at tip DDMN can deliver drug into dermis with minimal accumulation at stratum corneum.
DDMN has 86% delivery efficiency.
Model antigen OVA embedded in DDMN is stable at least 35 days at room temperature.
At the same dose, DDMN produces 10-fold stronger anti-OVA responses compared to IM vaccination.
The DDMN matrix could improve the denaturation temperature of the OVA from around 70-120 °C to over 150 °C, as demonstrated by differential scanning calorimetric analysis. In vivo delivery of OVA antigen into the mice’s skin via DDMN elicited 10 times higher specific antibody responses compared to conventional intramuscular injection. We envision DDMN as an effective, precise dosing, intradermal vaccine delivery system that may require no cold-chain, offers a dose-sparing effect, and can be administered easily.
1. Introduction
Vaccines serve as one of the most cost-effective strategies for the eradication or reduction of diseases that cause severe illness or death from infection. Vaccines are designed to generate protective immune responses and prevent disease from spreading. To date, the common types of vaccines include live attenuated organisms, killed vaccines, subunit vaccines, viral-like particles, and nucleic acid-base vaccines such as plasmid DNA and messenger RNA vaccines [1], [2]. Subunit vaccines, which consist of purified parts of an organism, are one of the most anticipated new vaccines. This is not only because removing non-essential parts of the organism can reduce the risk of autoimmune or unwanted inflammatory reactions compared to live attenuated or killed vaccines, but also because it lacks genetic material [3], [4]. However, subunit vaccines are inferior to other types of vaccines in terms of immunogenicity and therefore require a formulation with a proper adjuvant [5]. Nevertheless, the use of adjuvants comes with toxicity and adverse side effects [6]. By selecting an appropriate route of administration, it is possible to increase the immunogenicity of the subunit vaccine without incurring additional adverse effects [6], [7]. With abundant antigen-presenting cells, including epidermal Langerhans cells and dermal dendritic cells, at the epidermis-dermis junction, intradermal administration of the vaccine can increase its immunogenicity in comparison to the more commonly administered muscle site [8], [9], [10]. Numerous human studies have proven the validity of this theory [11]. However, not only is it difficult to inject liquid vaccine solution into this thin layer underneath the skin’s surface, but acute pain is unavoidable [10], [12]. Without a skilled professional, it would be easy to miss a site or induce bruising or blistering [13], [14], [15].
Although dissolvable microneedles (DMNs) have long been proposed as a minimally invasive, painless, and easy-to-administer vaccine delivery device with potential benefits in increased immunogenicity and antigen stability to the point where they may not require a cold chain, this device still faces challenges in terms of dose accuracy and delivery reliability [16], [17], [18], [19]. The issues associated with dose accuracy and delivery reliability originate from the prerequisites for two contradictory device characteristics: consistent sharpness of the needles for unfailing skin penetration and rapid dissolution of the needles under a limited amount of water in the skin for complete dose delivery. Needles with a fast dissolution rate in a limited water environment, however, can easily absorb moisture from the air and turn soft, resulting in poor skin penetration. One approach is to use microneedles that can retain the needle’s sharpness but have a slower dissolution rate and are used at a longer administration time. However, this results in other serious issues, such as the unpredictable and difficult-to-notice rebound of the needles from the skin during the extended wearing period and the prolonged opening of the skin at the needle-puncturing points [20], which inhibits the natural rapid resealing process of the skin.
We previously reported on detachable-dissolvable microneedles (DDMN) that enable needles to be detached from the base during administration without relying on the water content of the skin or the rapid dissolution rate of the needle materials [20], [21], [22], [23]. We report here details on 1) the development of the DDMN to have high precision in dosing and reliability in delivering cargoes into the skin; 2) the stabilization of the ovalbumin (OVA) subunit vaccine in the solid DDMN made from biocompatible materials that could alter the denaturation temperature of the protein cargo; and 3) the application of the DDMN to deliver the OVA to the dermis of the mice and the monitoring of their IgG subclass responses. All these three major factors led to our demonstration here of a subunit vaccine in the form of a disposable DDMN patch that not only has a 10-fold higher immunogenicity compared to traditional intramuscular administration but can also be given painlessly in two minutes with accurate dosing and can be kept at room temperature. The paper also includes discussions on the molecular mechanism of improved OVA stability when kept in DDMNs and the biological pathway of increased immunity via DDMN administration.
2.1. Materials
Sodium hyaluronate (HA; MW 5 kDa and 2000 kDa) was purchased from Baoding Faithful Industry (China). Trehalose, ovalbumin (OVA; from chicken egg white, MW 44.3 kDa), ponceau, and azorubine were purchased from Sigma-Aldrich (St Louis, MO). Anti-OVA (mouse monoclonal antibody clone TOSG1C6) and horseradish peroxidase (HRP)-labeled goat anti-mouse total IgG, IgG1, and IgG2 were purchased from BioLegend (CA, USA). DDMN iron master molds (the circular disk with a diameter of 1.15 cm containing 37 square nail-shaped needles arranged with a tip-to-tip distance of 1150 µm; each needle is nail-shaped with a 250, 250, 270 µm (W, L, H) square column and a 430 µm height of the square pyramid on the top. were from Mineed Technology (Thailand). Lint-free polyester sheet assembled with a sticker at the rim was obtained from BOYD Technologies (Thailand) and was subject to gamma sterilization before use. A commercial solution kit for making polydimethyl siloxane silicone rubber molds (food-grade platinum-cured) was purchased from Rungart Resin Company (Thailand).
Download the full study Pre-proof as PDF here: Detachable-Dissolvable-Microneedle as a potent subunit vaccine delivery device that requires no cold-chain
or read it here
Theerapat Phoka, Naruchit Thanuthanakhun, Peerapat Visitchanakun, Narintorn Dueanphen, Nisha Wanichwecharungruang, Asada Leelahavanichkul, Tanapat Palaga, Kiat Ruxrungtham, Supason Wanichwecharungruang, Detachable-Dissolvable-Microneedle as a potent subunit vaccine delivery device that requires no cold-chain, Vaccine: X, 2023, 100398, ISSN 2590-1362,
https://doi.org/10.1016/j.jvacx.2023.100398.
See the webinar:
“Rational Selection of Cyclodextrins for the Solubilization of Poorly Soluble Oral Drugs”, 8. November 2023:
Get more information & register here for free:


