Equivalence assessment of creams with quali-quantitative differences in light of the EMA and FDA regulatory framework
Abstract
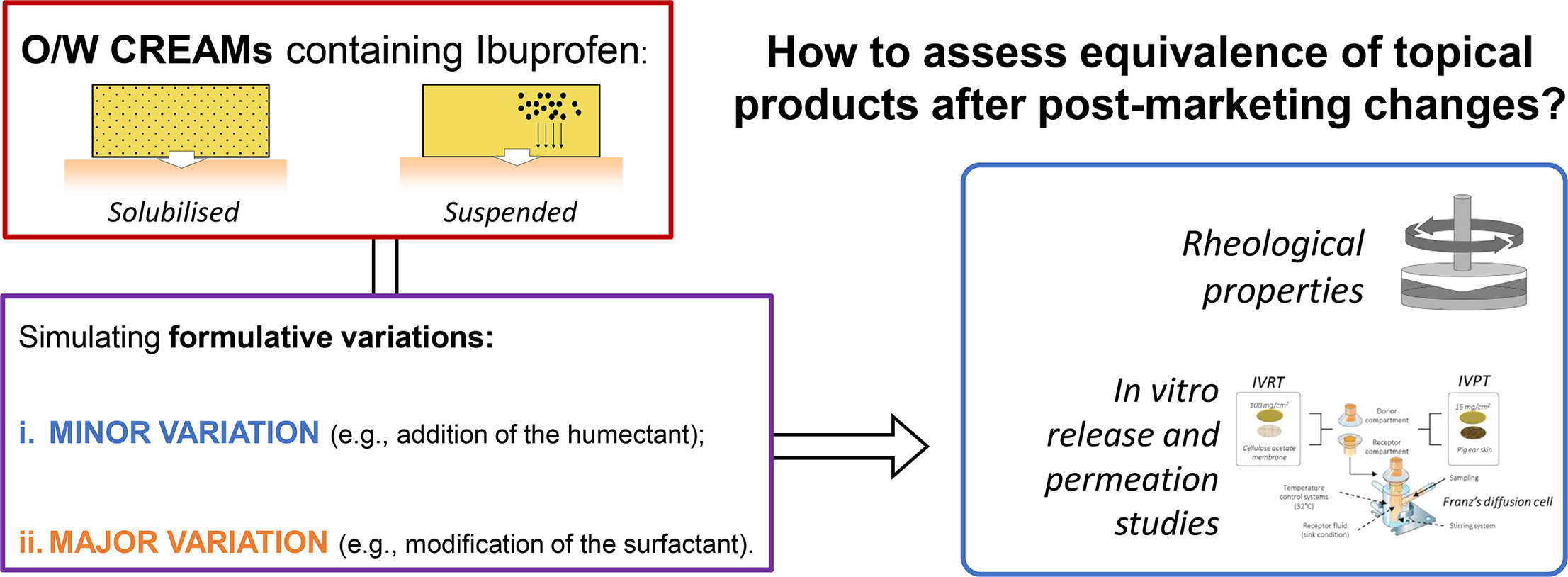
EMA and FDA are upgrading guidelines on assessing the quality and the equivalence of topically applied drug products for developing copies of originator products and supporting post-marketing variations. For topical products having remarkably similar composition, both EMA and FDA accept the equivalence on the bases of the comparison of rheological properties and in vitro drug release constant (k) and skin permeation flux (J) values, instead of clinical studies. This work aims to evaluate the feasibility to expand this approach to variations of the composition of complex semi-solid preparations. Ibuprofen (IB) creams at two different strengths (i.e., 1 % and 10 %) were used as a model formulation. Two formulative changes were performed: (a) the addition of the humectant to simulate a minor post-marketing variation; (b) the substitution of the emulsifying system to simulate a major one. These variations impacted only in 1 % IB formulations where both the equivalences of rheological data and J-values failed. At the highest concentration, the presence of IB crystals broke down the differences in rheological patterns and lead the IB thermodynamic activity at the maximum figuring out an overlapping of the J-values. Such data suggest the combination of these studies, which are thought mainly for the development of copies, could be also applied to the management of post-marketing variations that involve product composition.
Introduction
The development of a new locally acting product should take into consideration technological and regulatory constraints to ensure the final quality, efficacy, and safety. To speed up pharmaceutical development and prevent quality failures during the product shelf-life, there is a growing interest in the pharmaceutical industry to identify, earlier in the development phases, critical attributes able to affect the manufacturing process and, therefore, the product quality profile. This information contributes to the definition of the Quality Target Product Profile (QTPP), which is defined by the ICH Q8 (R2) guideline as “a prospective summary of the quality characteristics of a drug product that ideally will be achieved to ensure the desired quality, taking into account safety and efficacy of the drug product” (EMA, 2017). Critical Quality Attributes (CQA) of a locally acting semi-solid product may vary based on the features of the semi-solid preparation, and the physical state of the drug substance (e.g., drug-dissolved preparations versus drug-suspended ones) (Chang et al., 2013). In this context, a correct balance between the rheological characteristics of the semi-solid preparation, in terms of usability and physical stability, and the other functionality-related properties should be achieved to reach the target efficacy and safety profiles. Indeed, rheological properties impact not only the product texture properties, but also the drug release from the semi-solid matrix and, consequently, the drug absorption through the skin (Krishnaiah et al., 2014).
These aspects are particularly relevant in the development of generic products or when post-marketing variations of an authorized medicinal product are needed. The novel formulation (i.e., generic product, modified formula) should conform to the same QTPP of the reference product; therefore, product quality metrics are needed to prove therapeutic equivalence and interchangeability with their reference product (Krishnaiah et al., 2014; Minghetti et al., 2020). However, the in vivo quantification of drug concentration at the local level (e.g., skin) may require sophisticated techniques (Pudney et al., 2007); on the other side, after a local application, the plasmatic drug concentration results negligible in most cases. Therefore, the assessment of bioequivalence for locally acting products and/or follow-on products is challenging and may require clinical endpoint trials (Minghetti et al., 2020). To overcome such gaps, huge efforts have been made in develop and validating minimal invasive methodologies, such as microdialysis and open flow microperfusion, able to determine drug concentration in vivo directly in dermal layer (Birngruber et al., 2022). A parallel approach to waive clinical trials pursued by regulatory authorities is the release of guidance in which surrogate in vitro, and in vivo methods are detailed to assess equivalence (CFR, 2022; EMA, 2018; Miranda et al., 2018). For example, in vitro release tests (IVRT), permeation kinetic studies [e.g., in vitro permeation tests (IVPT), in vivo stratum corneum sampling (i.e. tape stripping)] or, if available, pharmacodynamic tests (e.g., skin blanching for glucocorticoids) have been accepted (CFR, 2022; EMA, 2018; FDA, 1997; Minghetti et al., 2020; Miranda et al., 2018). In particular, IVRT has been recognized as an excellent tool to evaluate the quality of a product formulation and to identify those differences between products that can impact on the drug release profile. On the contrary, IVPT allow an estimation of the impact of drug thermodynamic activity on skin absorption. Therefore, IVRT discriminate formulations based on their qualitative, quantitative, and microstructure attributes (Alves et al., 2021; Krishnaiah et al., 2014; Miron et al., 2021; Tiffner et al., 2021).
In this light, the SUPAC-SS guidance released by the FDA in 1997 remained for a long time the regulatory standard for assessing product equivalence in scaling-up and post-approval changes (FDA, 1997). However, the increased awareness of regulatory communities on relevance of microstructure/rheological patterns in assessing the therapeutic equivalence of semi-solid products (Al-Ghabeish et al., 2015; Krishnaiah et al., 2014; Miron et al., 2021; Navarro-Pujol et al., 2021; Xu et al., 2020) pushed regulatory authorities towards novel orthogonal regulatory approaches. In 2018, the EMA proposes to use so-called extended pharmaceutical equivalence for comparing simpler formulations (e.g., ointments, and gels in which the drug is solubilized) (EMA, 2018). Based on such an approach, the therapeutic equivalence between two products should be based on the sameness of the pharmaceutical form, method of administration, qualitative and quantitative composition, the microstructure/physical properties, and the product performance (e.g., IVRT). For more complex preparations (e.g., multiphase systems such as creams), a permeation kinetic study, or a pharmacodynamic one should be performed, at least, by the applicant. In parallel, the provisions of SUPAC-SS guidance (FDA, 1997) were also expanded by the FDA with a draft guidance in 2022 (FDA, 2022). The current FDA approach for equivalence assessment is built on three pillars: same qualitative (Q1), and quantitative (Q2) compositions, and structurally/functionally equivalence (Q3). The latter one involves not only IVRT, but also the rheological pattern at least. Such an approach has been supported by the extensive literature on Q1/Q2 equivalent formulations (Alves et al., 2021; García-Arieta et al., 2023; Ilić et al., 2021; Krishnaiah et al., 2014; Miranda et al., 2018; Xu et al., 2015). However, after SUPAC-SS exceeding, little is written about novel regulatory approaches that should be applied for assessing equivalence between products having significant differences in quali-quantitative composition, especially in excipients that might affect drug bioavailability and performance (e.g., surfactants, permeation enhancers). Such regulatory uncertainty appears particularly critical for marketing authorization holders (MAHs) that must introduce minor and major post-marketing variations in authorized semi-solid products to meliorate their quality patterns (e.g., better physical stability in real-world conditions) or to ensure the availability of product on the market. This last aspect is particularly true for severe shortages of raw materials or closure of suppliers, in which MAHs are forced to apply for minor or major post-marketing changes to minimize the negative ripple effects on their manufacturing and, consequently, on the supply of drug products on the market (Musazzi et al., 2020). For these conditions, it is evident that the availability of regulatory approaches to waive clinical trials for assessing products’ equivalence is extremely critical for the resilience of MAHs and the continuity of care.
This article aims to assess the structural and functional similarity of semi-solid preparations that are not equivalent in terms of qualitative and quantitative composition. Starting from a cetomacrogol base cream reported in the Italian Pharmacopeia (FU XII ed.), two formulative changes were made to simulate: (i) a minor post-marketing variation by addition of the humectant (EMA, 2018) and (ii) a major variation by substituting the emulsifying system. Such variations were adopted in two series of creams that differ for the thermodynamic activity of the loaded drug [i.e., ibuprofen (IB)]: unknown, when IB is solubilized in the semi-solid matrix, and maximal, when it is partially suspended. The final composition of each formulation was adjusted by varying the water content to obtain within the same series of creams the same value of viscosity determined by a Brookfield apparatus. The formulations were then compared in terms of IVRT, IVPT, and rheological pattern. In this latter case, each formulation was characterized by determining the complete flow curve of viscosity versus shear rate, yield stress and linear viscoelastic response.
Download the full article as PDF here Equivalence assessment of creams with quali-quantitative differences in light of the EMA and FDA regulatory framework
or read it here
Materials
Ibuprofen (IB) was provided by Farmalabor (Italy), Cetostearyl Alcohol, Cetomacrogol 1000, White Soft Paraffin BP-USP were supplied by A.C.E.F. (Italy). Sepineo™ SE68 (Cetearyl alcohol/Cetearyl glucoside) was acquired from SEPPIC (France), and Paraffin oil was purchased from Carlo Erba Reagents (Italy). Glycerol, Polyethylene glycol 400, HPLC grade acetonitrile, cellulose acetate hydrophilic 0.45 µm membrane and nylon 0.22 µm filter were provided by VWR Chemicals (Belgium). The PVDF hydrophilic membrane was supplied by GVS (USA).
Paola Volontè, Umberto M. Musazzi, Luca Arnaboldi, Marco A. Ortenzi, Antonella Casiraghi, Francesco Cilurzo, Paola Minghetti, Equivalence assessment of creams with quali-quantitative differences in light of the EMA and FDA regulatory framework, European Journal of Pharmaceutical Sciences, Volume 195, 2024, 106726, ISSN 0928-0987,
https://doi.org/10.1016/j.ejps.2024.106726.
Read also our introduction article on Topical Excipients here:


