Sustained Release of Salicylic Acid from Ethyl Cellulose Microspheres Fabricated Using Quasi-Emulsion Solvent Diffusion Method
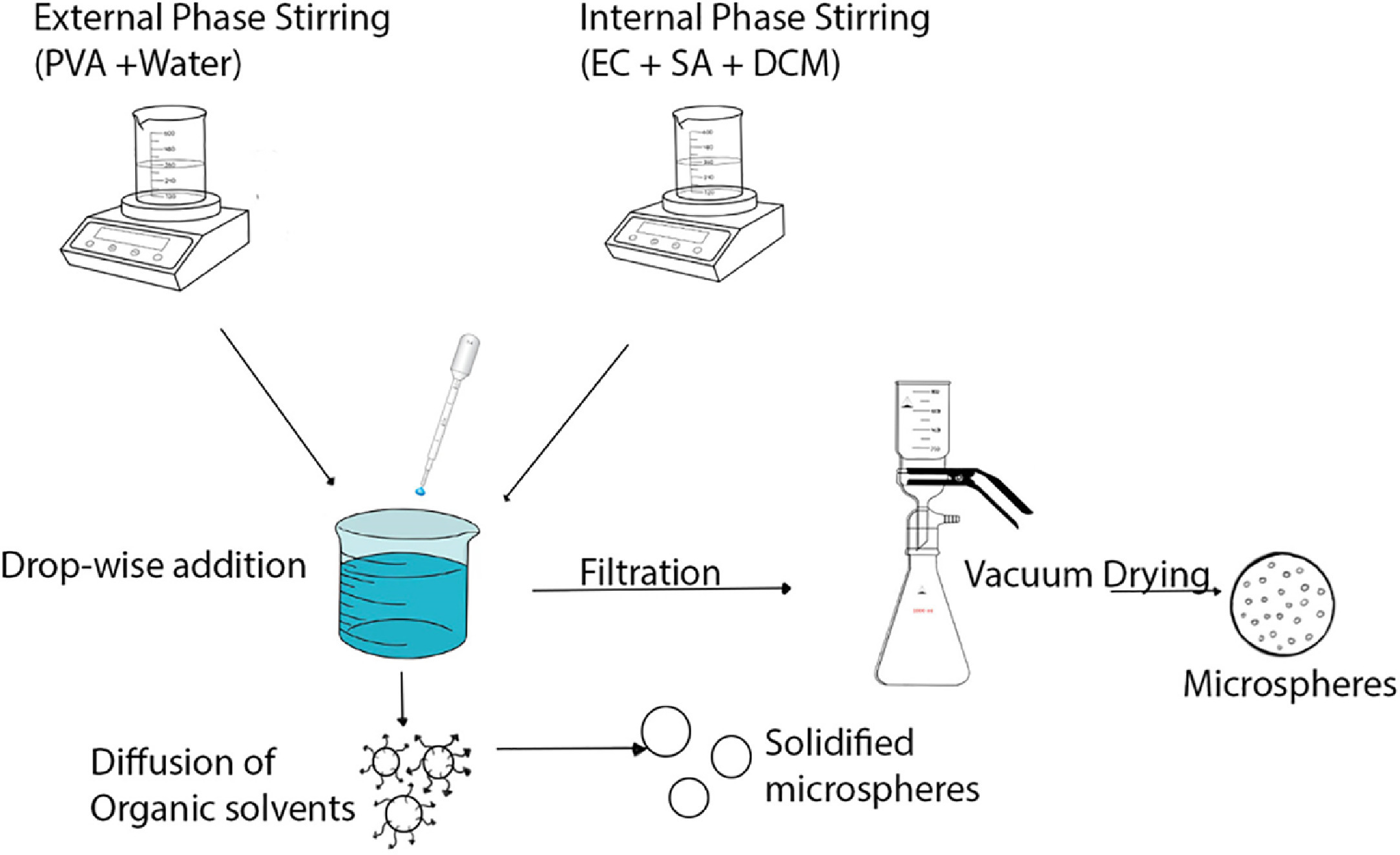
Delivery of drugs using micro-sized particles is an efficient tool. Using ethyl cellulose (EC) as a polymer and the quasi-emulsion solvent diffusion (QESD) method paves the way for the utilization of a cost-effective polymer and an efficient fabrication method. Poly(vinyl alcohol) in water was used as an external phase while EC and drug dissolved in dichloromethane as an internal phase. Salicylic acid (SA) is known to inhibit prostaglandins and have an anti-inflammatory effect. Furthermore, it inhibits the formation of bacterial biofilm on surfaces. Microparticles of several variations were successfully fabricated using the QESD method in an efficient time frame. These microparticles ranged in size from 5-40 μm for these formulations. Characterization results substantiated the selection of materials used for fabrication. Cytotoxicity showed that these fabrications were biocompatible and did not significantly inhibit cell proliferation.
In vitro drug release studies showed that the fabricated EC microparticles were able to sustainably release the drugs contained within them over a 96-hour time period, with most of the drug being released after 48 hours. This release never exceeded 63% of the total drug content. PEG addition in the fabrication process of formulation ECPSA eliminated burst release, resulting in a sustained release pattern over 48 hours, reaching saturation by day 5. While entrapment was dependent on the polymer content, increasing drug content did not significantly affect drug release. Behavioral studies evaluated the microspheres, revealing different kinetic models, including Higuchi in ECSA2 and Korsmeyer-Peppas in ECSA3, ECSA5, ECSA6, and ECPSA, with varying diffusion mechanisms. Future fabrications can be made to increase the porousness of the spheres to increase drug release from the microparticles. Then, drug release studies can be conducted over longer periods of time, and with nearly infrared light (NIR) to observe the changes in drug release between the fabrications.
2.1. Materials
Poly(vinyl alcohol) (PVA) (87-90% hydrolyzed with an avg. molecular weight of 30,000-70,000), dichloromethane (DCM), Ethyl Cellulose (EC) (48.0-49.5% (w/w) ethoxyl basis), and Salicylic Acid (SA) (practical grade ≥ 99.0%) were obtained from Millipore Sigma, USA. Ag NPs were purchased from Sky Nanomaterials, USA. Human embryonic kidney cells (HEK293) and poly(ethylene glycol) (PEG) (MW: 8000) were supplied by the Bioengineering Department, University of Massachusetts, Dartmouth. All chemicals used were of analytical grade without further purification.
Download the full study as PDF here: Sustained Release of Salicylic Acid from Ethyl Cellulose Microspheres Fabricated Using Quasi-Emulsion Solvent Diffusion Method
or read it here
Mishal Pokharel, Md Faiyaz Jamil, Jillian Pompei Wilson, Tracie Ferreira, Qinguo Fan, Kihan Park, Sustained Release of Salicylic Acid from Ethyl Cellulose Microspheres Fabricated Using Quasi-Emulsion Solvent Diffusion Method, Biomedical Engineering Advances, 2023, 100095, ISSN 2667-0992,
https://doi.org/10.1016/j.bea.2023.100095.

