Mucoadhesive 3D printed vaginal ovules to treat endometriosis and fibrotic uterine diseases
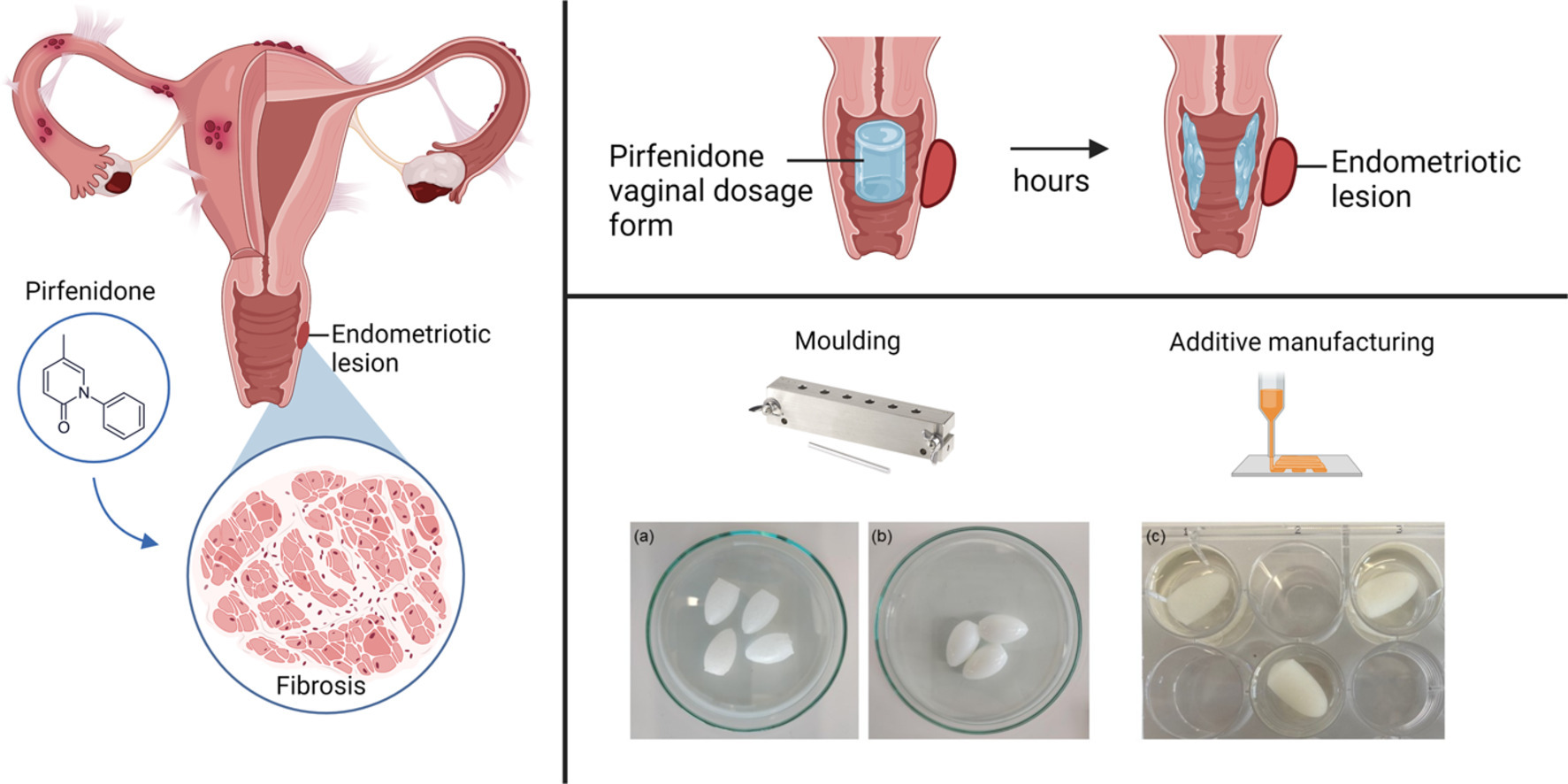
Gynaecological health is a neglected field of research that includes conditions such as endometriosis, uterine fibroids, infertility, viral and bacterial infections, and cancers. There is a clinical need to develop dosage forms for gynecological diseases that increase efficacy and reduce side effects and explore new materials with properties tailored to the vaginal mucosa and milieu. Here, we developed a 3D printed semisolid vaginal ovule containing pirfenidone, a repurposed drug candidate for endometriosis. Vaginal drug delivery allows direct targeting of the reproductive organs via the first uterine pass effect, but vaginal dosage forms can be challenging to self-administer and retain in situ for periods of more than 1–3 h. Here, we show that a semisoft alginate-based vaginal suppository manufactured using semisolid extrusion additive manufacturing is superior to vaginal ovules made using standard excipients.
Highlights
- Vaginal drug delivery of pirfenidone, a repurposed candidate for endometriosis
- Semisoft 3D printed mucoadhesive ovule at a dose of 120 mg pirfenidone per ovule
- Pharmacopeial, in vitro release and ex vivo mucoadhesion assays for vaginal ovules
- 3D printed ovule showed sustained release and mucoadhesive properties
- 24 h exposure of endometriotic cells to pirfenidone required for therapeutic action
The 3D-printed ovule showed a controlled release profile of pirfenidone in vitro in standard and biorelevant release tests, as well as better mucoadhesive properties ex vivo. An exposure time of 24 h of pirfenidone to a monolayer culture of an endometriotic epithelial cell line, 12Z, is necessary to reduce the cells’ metabolic activity, which demonstrates the need for a sustained release formulation of pirfenidone. 3D printing allowed us to formulate mucoadhesive polymers into a semisolid ovule with controlled release of pirfenidone. This work enables further preclinical and clinical studies into vaginally administered pirfenidone to assess its efficacy as a repurposed endometriosis treatment.
Download the full article as PDF here Mucoadhesive 3D printed vaginal ovules to treat endometriosis and fibrotic uterine diseases
or read it here
Chemicals
Pirfenidone (5-methyl-1-phenylpyridin-2(1H)-one) was purchased from BLD Pharm, Germany. Agarose, sodium alginate (product number W201502, quality level 400, viscosity of 5.0–40.0 cps in a 1% solution in water at 25°C), and hydroxypropylmethylcellulose (HPMC, product number 423203, molecular mass ∼86,000, viscosity of 2663–4970 cps in a 2% solution in water) were purchased from Sigma Aldrich, USA. Sodium acetate, acetic acid, trifluoroacetic acid (TFA), calcium chloride, HEPES, and phosphate buffered saline (PBS) were purchased from Carl Roth, Germany. Witepsol® S 58 was kindly gifted by IOI Oleo, Germany. Caffeine, glucose monohydrate, lactic acid, Macrogol 400, and Macrogol 1500 were purchased from Hänseler, Switzerland. Acetonitrile was purchased from Thermo Fisher Scientific, USA. Sodium lactate solution 50% was purchased from VWR, USA. Ultrapure water with a resistivity of 18.2 MΩ⋅cm was produced by a Barnstead Smart2pure device (Thermo Fisher Scientific).
Sarah Teworte, Simone Aleandri, Jessica Weber, Marianna Carone, Paola Luciani, Mucoadhesive 3D printed vaginal ovules to treat endometriosis and fibrotic uterine diseases, European Journal of Pharmaceutical Sciences, 2023, 106501, ISSN 0928-0987,https://doi.org/10.1016/j.ejps.2023.106501.
Read more on Overview of Pharmaceutical 3D printing here:


