The impact of diluents on the compaction, dissolution, and physical stability of amorphous solid dispersion tablets
Abstract
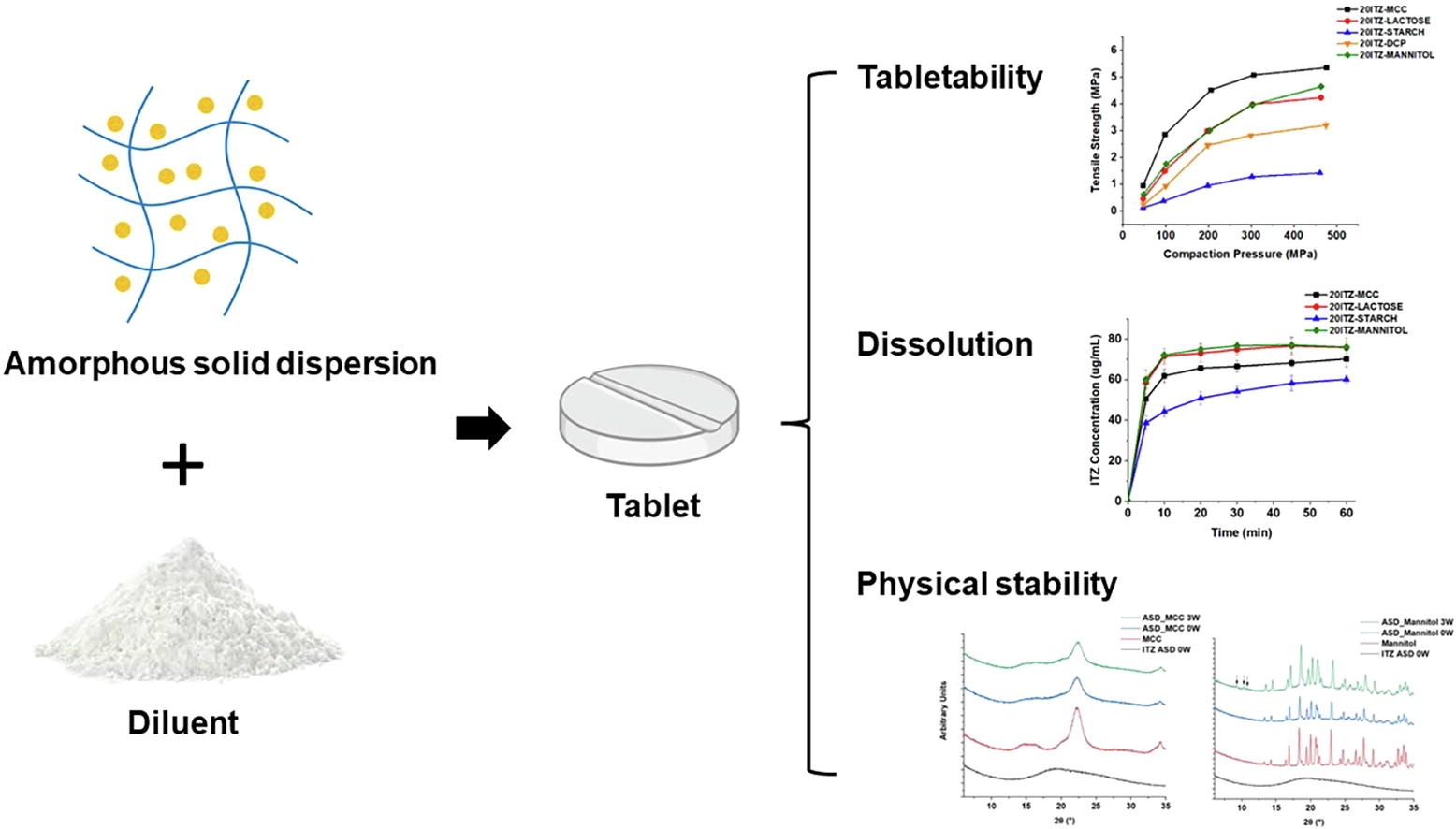
Amorphous solid dispersion (ASD) is an effective approach for enhancing the solubility, dissolution, and bioavailability of poorly water-soluble drugs. However, these metastable forms can transform into more thermodynamically stable, less soluble crystalline forms. Despite this challenge, research on processing ASDs into solid dosage forms, such as tablets, is lacking. This research paper aims to fill this gap by investigating the impact of common diluents on the tableting behavior, dissolution, and physical stability of ASDs composed of itraconazole and hypromellose acetate succinate. Four widely used diluents found in commercially available ASD tablets were selected for the study: microcrystalline cellulose (MCC), anhydrous lactose, starch, and mannitol. The performance of ASD tablets varied significantly depending on the diluent used. Notably, tablets prepared with MCC exhibited higher mechanical strength than those formulated using other diluents. ASD tablets containing mannitol and lactose revealed a faster release rate than those composed of MCC or starch. Furthermore, the study highlighted that the physical stability of ASDs within a tablet is not solely dependent on the amount of sorbed water; crystalline diluents like lactose and mannitol were found to facilitate ASD recrystallization within a tablet. In summary, the study underscores the importance of excipient selection, considering factors such as mechanical strength, dissolution rate, and physical stability of ASD tablets. These findings offer valuable insights into the selection of excipients for downstream ASD tablet development, leading to improved manufacturability, physical stability, and the overall quality of ASD drug products.
Introduction
Amorphous solid dispersions (ASDs) have been employed as a promising formulation strategy to enhance the solubility and subsequent oral bioavailability of poorly water-soluble drugs (Vasconcelos et al., 2007). One efficient and scalable method for preparing ASDs is spray drying, a solvent evaporation technique known for its reproducibility and continuous operation (Paudel, 2013, Sosnik and Seremeta, 2015). From spray-dried dispersions, various dosage forms can be developed, including tablets, capsules, and suspensions. Tablets, in particular, constitute over half of the FDA-approved ASD products (Bhujbal, 2021), with notable examples like Pifeltro® (doravirine tablet), Intelence® (etravirine tablet), and Kalydeco® (ivacaftor tablet). Tablets offer several advantages, including high patient compliance, portability, relatively low manufacturing costs, and ease of scale-up (Garg, 2010). However, ASDs generated through lab-scale spray dryers often exhibit undesirable characteristics, such as small particle size, low bulk density, and poor flowability, which can impede tableting processes (Honick, 2020). Additionally, maintaining the physical stability of the amorphous active pharmaceutical ingredient (API) throughout downstream processing and storage remains a key concern in ASD product development. Therefore, meticulous excipient selection and process design during the development of tablet formulations are essential.
In addition to the API(s), a typical tablet formulation consists of multiple excipients, each serving a unique function. Among these, diluents play a crucial role in increasing the bulk of the tablet and addressing potential issues associated with the API, such as inadequate flowability, poor compaction properties, and capping. Commonly used diluents include microcrystalline cellulose (MCC), starch, mannitol, dibasic calcium phosphate, lactose, and sucrose. For instance, previous research has shown that diluents with high compressibility, such as MCC, display exceptional plasticity and minimal elasticity, resulting in enhanced compaction properties (DiNunzio, 2012). Our prior research revealed that spray-dried dispersions often experience lamination at high compression speeds due to their pronounced elastic recovery and ejection force (Honick, 2020). To overcome this issue, a diluent, such as lactose, is necessary to decrease elastic recovery and residual die wall pressure in the formulation (Abdel-Hamid et al., 2012, Tanner, 2018). Additionally, some diluents, like starch, exhibit a self-lubricating property and have the potential to reduce the ejection force of the formulation (Abdel-Hamid et al., 2012).
Aside from enhancing the compaction process during ASD tablet production, an ideal diluent should possess qualities such as inertness, adequate solubility, and excellent compatibility with other components. These attributes are crucial for ensuring stability against drug recrystallization and optimal release behavior, both vital aspects for amorphous formulations (Yu, 2001). Studies have revealed instances of diluent-induced API recrystallization compromising tablet dissolution. For example, amorphous ibipinabant tablets stored at 40 °C/75 % relative humidity (RH) experienced API recrystallization induced by mannitol and lactose after three months (Leane, 2013). A similar trend was observed with melt-quenched amorphous nifedipine, where mannitol accelerated drug nucleation under desiccated conditions (Zhang, 2014). Another study showed that using MCC in PVP ASD capsules resulted in inferior drug release compared to lactose, attributed to the formation of a hard plug (Fakes, 2009).
As evident from the information presented, the rational selection of diluents is critical in ASD tablet development. This research paper aims to shed light on the significance of excipient selection in tablet formulation by thoroughly examining the effects of individual diluents on the compaction behavior, dissolution performance, and physical stability of ASD tablets.
Based on the solubility of the diluents, they can be classified into three groups: (i) poorly soluble diluents, such as celluloses and calcium salts; (ii) water-soluble diluents, such as mannitol and lactose; and (iii) partially soluble diluents, such as pregelatinized maize starch (Rane et al., 2010). In this study, four diluents – MCC, lactose anhydrous, mannitol, and starch – were chosen as they are commonly used in commercialized ASD tablets (Table 2). All diluent grades were suitable for direct compression.
In the current study, the model ASD consists of itraconazole (ITZ) and hypromellose acetate succinate (HPMCAS) L grade. ITZ is weak base (pKa = 3.7, log P = 5.7) with poor aqueous solubility (<5 ng/mL at 25 °C), classified to BCS Class II (Information, 2021). Additionally, ITZ falls under Developability Classification System class IIb (Kourentas, 2016), indicating that its oral absorption is primarily hindered by low aqueous solubility rather than slow dissolution rate. Consequently, amorphization, as opposed to methods such as particle size reduction to enhance dissolution, is likely a more effective strategy for improving ITZ dissolution and subsequent oral absorption. HPMCAS, a frequently employed polymer in ASDs, serves as a crystallization inhibitor by constraining molecular motion and elevating the overall glass transition temperature (Tg) of the ASD (Honick, 2020).
The model tablet formulations consisted of 47.75 % (w/w) ITZ-ASD, 47.75 % of the selected diluent, 4 % of sodium starch glycolate (SSG) as the disintegrant, and 0.5 % of magnesium stearate (MgSt) as the lubricant. A compaction simulator was employed to assess the compaction behavior of each formulation. Dissolution testing of the tablets was conducted at pH 6.8. All tablets were stored at 40 °C/75 % RH, and powder X-ray diffractometry (PXRD) as utilized to detect any potential crystallization of the ASDs. It is worth noting that ASDs with two different drug loadings were prepared in this study to clearly illustrate the differences and support our findings. For all the dissolution studies, we used ASDs with a 20 % drug loading based on preliminary data that indicated the desired drug release for ITZ-HPMCAS ASDs with low drug loadings (Friesen, 2008, Saboo, 2019); while for the stability study, we selected an ASD with an 80 % drug loading to examine the effects of diluents within a reasonable experimental timeframe. This decision was based on our preliminary study, which demonstrated the high stability of ITZ-ASDs with a high polymer content (data not shown) (Table 3).
Read more here
Materials
Itraconazole (USP grade) was received from Letco Medical Inc (Decatur, AL). HPMCAS L grade (AquaSolve™) was acquired from Ashland (Wilmington, DE). MCC (Avicel® PH-102) was obtained from FMC Corporation (Philadelphia, PA). α-Lactose anhydrate (SuperTab® AN) and magnesium stearate were purchased from Sigma-Aldrich (St. Louis, MO). Mannitol (Pearlitol® 200 SD) and sodium starch glycolate (GLYCOLYS®) were gifted by Roquette (Lestrem, France). Starch 1500 from Colorcon (West Point, PA). The ASD were dry granulated by creating large slugs with a STYL’One Evo compaction simulator (MEDELPHARM, Beynost, France) equipped with a flat round 16 mm diameter tooling.
Dongyue Yu, Stephen W. Hoag, The impact of diluents on the compaction, dissolution, and physical stability of amorphous solid dispersion tablets, International Journal of Pharmaceutics, 2024, 123924, ISSN 0378-5173, https://doi.org/10.1016/j.ijpharm.2024.123924.
Read also our introduction article on Mannitol here:


