High-Shear Wet Granulation of SMEDDS Based on Mesoporous Carriers for Improved Carvedilol Solubility
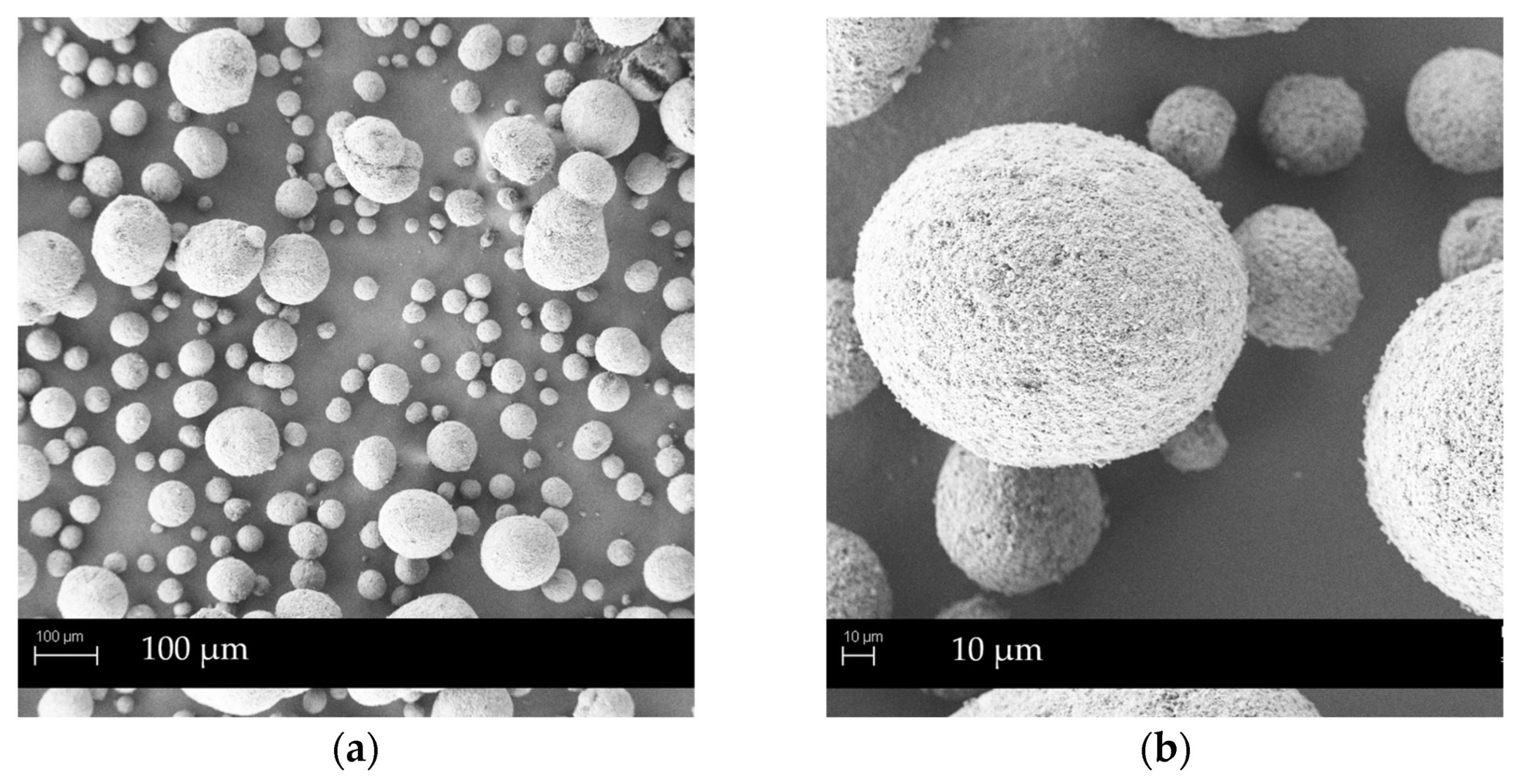
Mesoporous carriers are a convenient choice for the solidification of self-microemulsifying drug delivery systems (SMEDDS) designed to improve the solubility of poorly water-soluble drugs. They are known for high liquid load capacity and the ability to maintain characteristics of dry, free-flowing powders. Therefore, five different mesoporous carriers were used for the preparation of carvedilol-loaded SMEDDS granules by wet granulation methods—in paten (manually) and using a high-shear (HS) granulator.
Granules with the highest SMEDDS content (63% and 66% of total granules mass, respectively) and suitable flow properties were obtained by Syloid® 244FP and Neusilin® US2. SMEDDS loaded granules produced by HS granulation showed superior flow characteristics compared to those obtained manually. All SMEDDS granules exhibited fast in vitro release, with 93% of carvedilol releasing from Syloid® 244FP-based granules in 5 min. Upon compaction into self-microemulsifying tablets, suitable tablet hardness and very fast disintegration time were achieved, thus producing orodispersible tablets. The compaction slightly slowed down the carvedilol release rate; nevertheless, upon 1 h (at pH 1.2) or 4 h (at pH 6.8) of in vitro dissolution testing, the amount of released drug was comparable with granules, confirming the suitability of orodispersible tablets for the production of the SMEDDS loaded single unit oral dosage form.
Download the full article as PDF here High-Shear Wet Granulation of SMEDDS Based on Mesoporous Carriers for Improved Carvedilol Solubility
or read it here
Materials
Carvedilol (CTX Life Sciences Ltd., Gujarat, India) was used as a model drug.
Liquid SMEDDS was composed of Capmul MCM EP/NF (mono-diglyceride of medium chain fatty acids, Abitec Corporation, Columbus, OH, USA), refined castor oil (Ph. Eur. Grade, Caesar & Loretz GmbH, Hilden, Germany), Kollisolv PEG E 400 (Sigma-Aldrich, St. Louis, MO, USA) and Kolliphor RH 40 (polyoxyl 40 hydrogenated castor oil, Sigma-Aldrich, USA).
Five different mesoporous carriers were used for SMEDDS granulation: Syloid 244 FP (silica with average particle size 3.5 μm, Grace GmbH & Co. KG, Worms, Germany), Fujicalin SG (SA) (anhydrous calcium hydrogen phosphate with mean particle size 120 μm, Fuji Chemical Industries Co. Ltd., Toyama, Japan), Neusilin US2 (amorphous magnesium aluminometasilicate with mean particle size 106 μm, Fuji Chemical Industries Co. Ltd., Japan), Syloid XDP 3050 (silica with average particle size 50 μm, Grace GmbH & Co. KG, Germany), and Aeroperl 300 (fumed silica with average particle size 20–60 μm, EVONIK, Essen, Germany). Povidone K25 (Kollidon 25) was used as a binder in granulation dispersion (GD).
For SMEDDS tablet production, additional excipients were used, specifically copovidone VA64 (Kollidon VA64, BASF, Ludwigshafen, Germany) as a binder, crosscarmellose sodium (Ac-Di-Sol, FMC BioPolymer, Philadelphia, PA, USA) as a disintegrant, microcrystalline cellulose (Avicel PH 200, FMC Biopolymers, USA) as a filler, and magnesium stearate (Merck KGaA, Darmstadt, Germany) as a lubricant and antiadhesive agent. For preparation of dissolution media, KH2PO4 (Merck KGaA, Germany), NaOH (Merck KGaA, Germany), HCl 37% (Panreac Quimica S.A.U., Barcelona, Spain) and purified water (reverse osmosis, Faculty of Pharmacy, Ljubljana, Slovenia) were used.
Kovačević, M.; German Ilić, I.; Bolko Seljak, K.; Zvonar Pobirk, A. High-Shear Wet Granulation of SMEDDS Based on Mesoporous Carriers for Improved Carvedilol Solubility. Pharmaceutics 2022, 14, 2077. https://doi.org/10.3390/pharmaceutics14102077

