W/O/W microemulsions for nasal delivery of hydrophilic compounds: a preliminary study
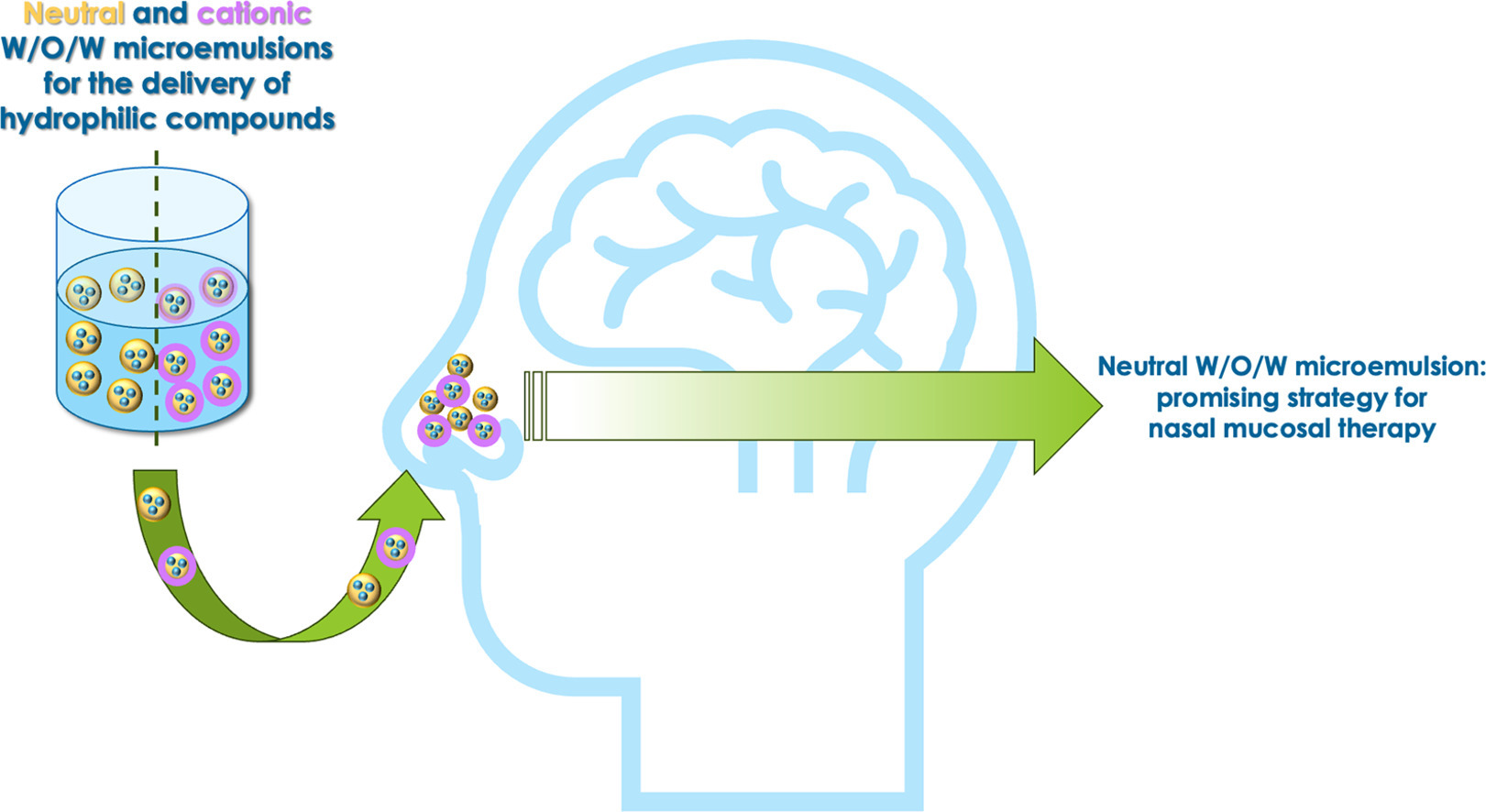
The administration of hydrophilic therapeutics has always been a great challenge because of their low bioavailability after administration. For this purpose, W/O/W microemulsion resulted to be a potential successful strategy for the delivery of hydrophilic compounds, interesting for the nasal mucosal therapy. Herein, an optimized biphasic W/O microemulsion was designed, through a preliminary screening, and it was inverted in a triphasic W/O/W microemulsion, intended for the nasal administration. In order to enhance the mucosal retention, surface modification of the biphasic W/O microemulsion was performed adding didodecyldimethylammonium bromide, and then converting the system into a cationic triphasic W/O/W microemulsion. The developed samples were characterized in terms of droplet size, polydispersity, zeta potential, pH and osmolality.
Highlights
- The preformulative screening allowed the development of W/O/W microemulsions adequate for the intended nasal administration.
- Accelerated stability studies demonstrated a long-term stability for the prepared W/O/W microemulsions.
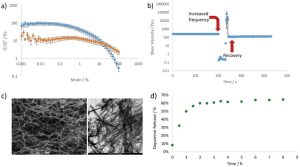
- W/O/W microemulsions showed good mucoadhesive properties, which could guarantee the adhesion on the nasal mucosa.
- A rapid and sustained release of fluorescein from the neutral W/O/W microemulsion was obtained.
- Neutral W/O/W microemulsion represents a promising system due to its high biocompatibility on HFF1 and Calu-3 cell lines.
The physical long-term stability was analyzed storing samples at accelerated conditions (40±2°C and 75±5% RH) for 6 months in a constant climate chamber, following ICH guidelines Q1A (R2). In order to verify the potential retention on the nasal mucosa, the two triphasic systems were analyzed in terms of mucoadhesive properties, measuring the in vitro interaction with mucin over time. Furthermore, fluorescein sodium salt was selected as a model hydrophilic drug to be encapsulated into the inner core of the two triphasic W/O/W microemulsions, and its release was analyzed compared to the free probe solution. The cytocompatibility of the two platforms was assessed on two cell lines, human fibroblasts HFF1 and Calu-3 cell lines, chosen as pre-clinical models for nasal and bronchial/tracheal airway epithelium.
Download the full article as PDF here W/O/W microemulsions for nasal delivery of hydrophilic compounds: a preliminary study
or read it here
Materials
Kolliphor® RH40 was provided by BASF Italia S.p.a. (Cesano Modena, Italy); Oleoyl Macrogol-6 Glycerides (Labrafil®) was a gift from Gattefossé Italia s.r.l. (Milano, Italy); Isopropyl myristate (IPM) and Triglyceride caprylic-capric (Tegosoft CT, Miglyol 812) were purchased from Farmalabor (Canosa di Puglia, Italy). Tween® 80 (Polysorbate 80), Span® 80 (Sorbitan monooleate), Didodecyldimethylammonium bromide (DDAB), Fluorescein sodium salt, Tris (hydroxymethyl)aminomethane buffer, Phosphate Buffered Saline pH 7.4 (PBS) were bought from Merck (Darmstadt, Germany). Mucin (mucin from porcine stomach type II), NaCl, NaHCO3, CaCl2·2H2O and KCl were purchased from Merck. Regenerated cellulose membranes (Spectra/Por CE; Mol. Wet. Cutoff 3500) were supplied by Spectrum (Los Angeles, CA, USA). All materials for the biological assays were purchased from Merck. All solvents (LC grade) were from VWR International (Milan, Italy).
Cinzia Cimino, Angela Bonaccorso, Barbara Tomasello, Giovanni Anfuso Alberghina, Teresa Musumeci, Carmelo Puglia, Rosario Pignatello, Agostino Marrazzo, Claudia Carbone, W/O/W microemulsions for nasal delivery of hydrophilic compounds: a preliminary study, Journal of Pharmaceutical Sciences, 2024, ISSN 0022-3549, https://doi.org/10.1016/j.xphs.2024.01.013.
Read more article an Nasal Delivery here:
- Spray dried powders for nasal delivery
- Enhanced Delivery of Neuroactive Drugs via Nasal Delivery with a Self-Healing Supramolecular Gel