Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome: An Update and Progression
Abstract
Medical practitioners commonly use oral and parenteral dosage forms to administer drugs to patients. However, these forms have certain drawbacks, particularly concerning patients’ comfort and compliance. Transdermal drug delivery presents a promising solution to address these issues. Nevertheless, the stratum corneum, as the outermost skin layer, can impede drug permeation, especially for macromolecules, genetic materials, stem cells, and secretome. Microneedles, a dosage form for transdermal delivery, offer an alternative approach, particularly for biopharmaceutical products. In this review, the authors will examine the latest research on microneedle formulations designed to deliver genetic materials, stem cells, and their derivatives. Numerous studies have explored different types of microneedles and evaluated their ability to deliver these products using preclinical models. Some of these investigations have compared microneedles with conventional dosage forms, demonstrating their significant potential for advancing the development of biotherapeutics in the future.
Introduction
There are 33 cellular and gene therapy products that have been approved by the United States Food and Drug Administrator (FDA), with the majority of them being administered through parenteral injection [1]. The parenteral route has gained preference over common options, like oral administration. This is because peptides and proteins are susceptible to degradation in the gastrointestinal tract and undergo first-pass metabolism in the liver [2]. The parenteral route remains the gold standard for these compounds [3]. Additionally, intravenous injection within the parenteral route ensures optimal (100%) drug bioavailability, as there is no absorption phase after administration [4]. As a result, the parenteral route has emerged as the primary choice for delivering cellular and genetic-based medical treatments [5]. However, the parenteral does come with its limitations. These include pain at the application site, the need for medical personnel for application, and the generation of needle waste [4,6,7]. Furthermore, cellular and gene therapy products often require multiple doses due to their short half lives. This frequent dosing can lead to poor patient compliance, as patients may find the treatments uncomfortable [5]. Additionally, peptide compounds in the form of liquid injections or lyophilised dosage forms require a cold storage condition. This presents challenges in terms of manufacturing facilities and cold chain distribution, particularly in tropical countries, like Indonesia [8]. Consequently, alternative delivery routes are needed to address these issues.
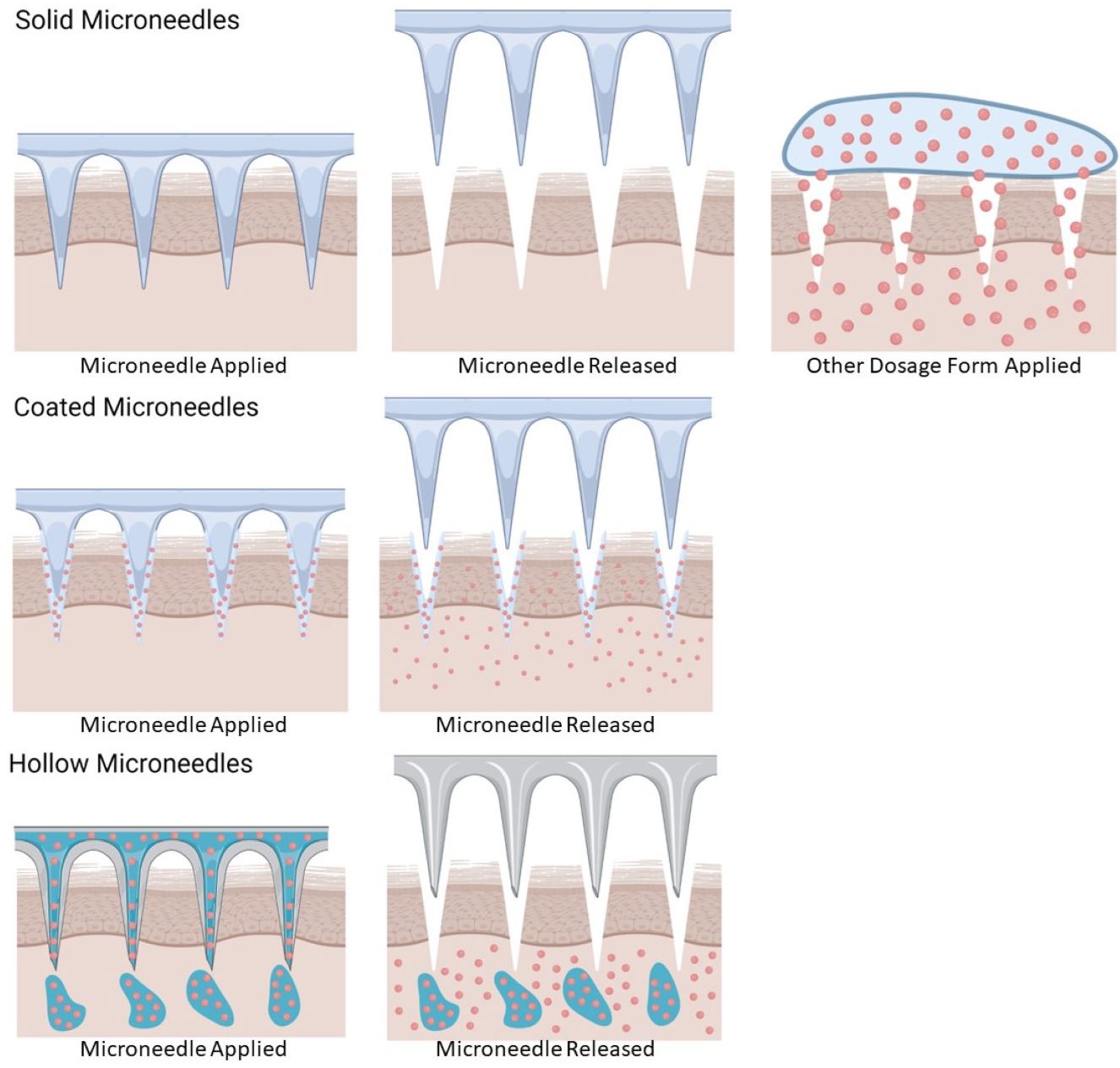
The transdermal route offers a potential solution to the challenges mentioned above. This approach is less invasive and pain-free compared to the parenteral route [6,9]. Transdermal drug delivery involves using healthy skin as the application site, allowing drugs to enter the systemic circulation through diffusion or percutaneous absorption [10]. The skin, being the body’s largest organ, provides a variety of application areas and is easily accessible to patients [11]. Given the presence of blood vessels and lymphatics in the dermal layer, transdermal drug delivery can still achieve systemic effects [11]. This review encapsulates the latest research on the microneedle-mediated transdermal delivery of genetic materials, stem cells, and their products.
Download the full article as PDF here: Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome
or read it here
Nainggolan, A.D.C.; Anjani, Q.K.; Hartrianti, P.; Donnelly, R.F.; Kurniawan, A.; Ramadon, D. Microneedle-Mediated Transdermal Delivery of Genetic Materials, Stem Cells, and Secretome: An Update and Progression. Pharmaceutics 2023, 15, 2767. https://doi.org/10.3390/pharmaceutics15122767
Read more Microneedle articles from this research group:
- Fabrication of dissolving microneedles for transdermal delivery of protein and peptide drugs
- Long-acting microneedle formulations
- Microneedle array patches for sustained delivery of fluphenazine