In Vitro Release, Mucosal Permeation and Deposition of Cannabidiol from Liquisolid Systems: The Influence of Liquid Vehicles
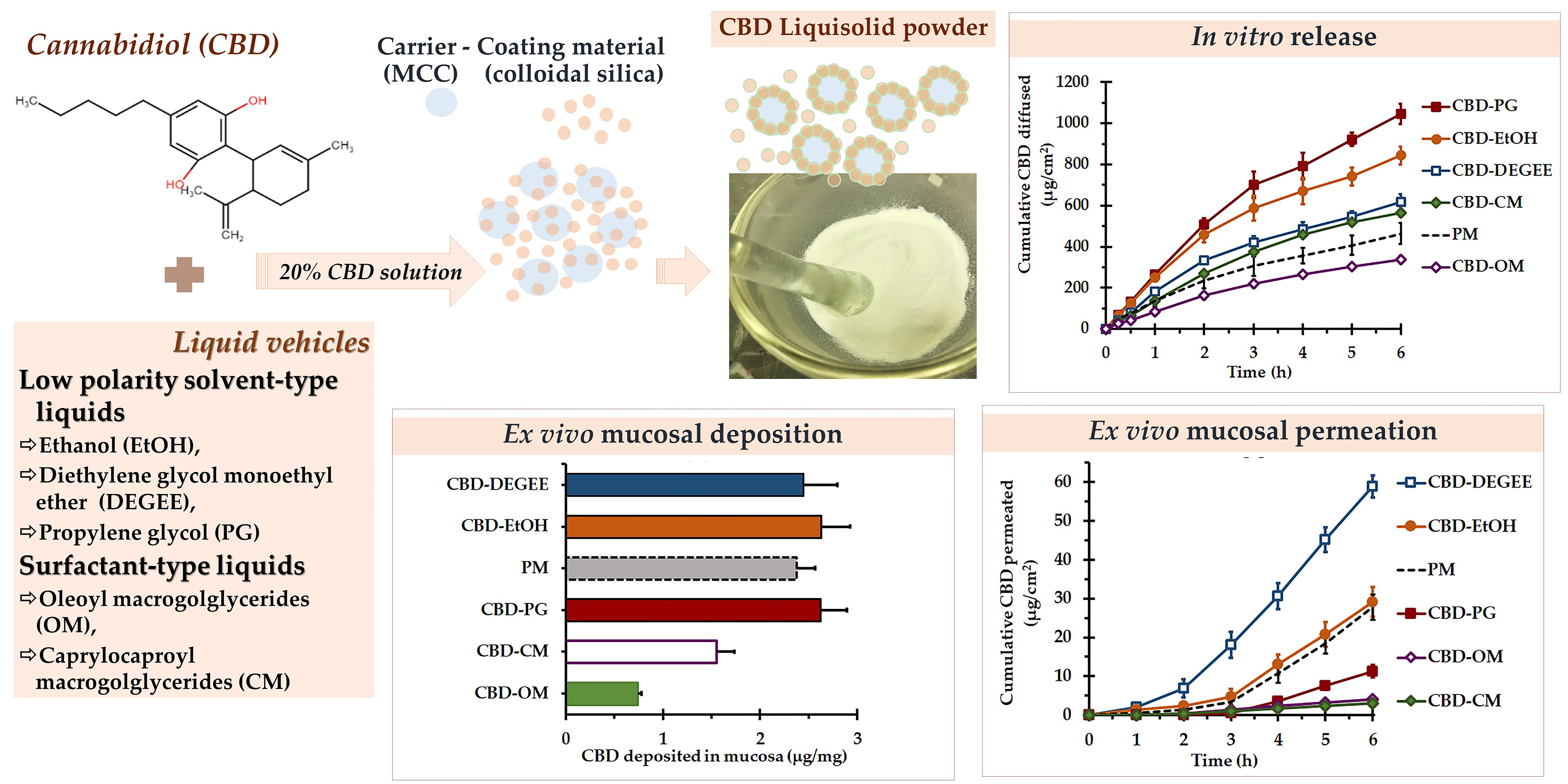
This work investigated the influence of liquid vehicles on the release, mucosal permeation and deposition of cannabidiol (CBD) from liquisolid systems. Various vehicles, including EtOH, nonvolatile low- and semi-polar solvents, and liquid surfactants, were investigated. The CBD solution was converted into free-flowing powder using carrier (microcrystalline cellulose) and coating materials (colloidal silica). A physical mixture of the CBD and carrier–coating materials was prepared as a control. The non-crystalline state of CBD in the liquisolid systems was confirmed using XRD, FTIR and SEM studies. The CBD liquisolid powder prepared with volatile and nonvolatile solvents had a better CBD release performance than the CBD formed as the surfactant-based and control powders. The liquisolid systems provided the CBD permeation flux through porcine esophageal mucosa ranging from 0.68 ± 0.11 to 13.68 ± 0.74 µg·cm−2·h−1, with the CBD deposition levels of 0.74 ± 0.04 to 2.62 ± 0.30 μg/mg for the dry mucosa. Diethylene glycol monoethyl ether showed significant CBD permeation enhancement (2.1 folds) without an increase in mucosal deposition, while the surfactants retarded the permeation (6.7–9.0 folds) and deposition (1.5–3.2 folds) significantly. In conclusion, besides the drug release, liquid vehicles significantly influence mucosal permeation and deposition, either enhanced or suppressed, in liquisolid systems. Special attention must be paid to the selection and screening of suitable liquid vehicles for liquisolid systems designed for transmucosal applications.
Download the full article as PDF here In Vitro Release, Mucosal Permeation and Deposition of Cannabidiol from Liquisolid Systems_The Influence of Liquid Vehicles
or read it here
Materials
Cannabidiol (CBD, CBD isolate, 99%) was kindly provided by the Medicinal Cannabis Research Institute, College of Pharmacy, Rangsit University (Pathumthani, Thailand). Microcrystalline cellulose (MCC; Avicel® PH102) and colloidal silicon dioxide (CSD, Aerosil® 200) were obtained from Onimax Co., Ltd. (Bangkok, Thailand) and Maxway Co., Ltd. (Bangkok, Thailand), respectively. Absolute ethyl alcohol (EtOH) was purchased from QRëC (Auckland, New Zealand). Caprylocaproyl macrogolglycerides (CM, Labrasol®, Gattefossé SAS (Saint-Priest, French)), diethylene glycol monoethyl ether (DEGEE, Transcutol® P, Gattefossé SAS (Saint-Priest, French)) and oleoyl macrogolglycerides (OM, Labrafil® M 1944 CS, Gattefossé SAS (Saint-Priest, French)) were provided by Rama Production, Co., Ltd. (Bangkok, Thailand). Glycerin and propylene glycol (PG) were sourced from RCI Labscan Ltd. (Bangkok, Thailand). Polyethylene glycol 400 (PEG) and polyoxyethylene sorbitan monolaurate (Polysorbate 20, P20) were provided by Sigma-Aldrich (Missouri, MO, USA) and AppliChem GmbH (Darmstadt, Germany), respectively. Formic acid was supplied by KemAus (Cherrybrook, Australia). HPLC grade methanol and acetonitrile were supplied by Fisher®Scientific (Loungborough, UK). All chemicals were used as received.
Tabboon, P.; Pongjanyakul, T.; Limpongsa, E.; Jaipakdee, N. In Vitro Release, Mucosal Permeation and Deposition of Cannabidiol from Liquisolid Systems: The Influence of Liquid Vehicles. Pharmaceutics 2022, 14, 1787. https://doi.org/10.3390/pharmaceutics14091787

