Solidifying Fenofibrate Nanocrystal Suspension: A Scalable Approach via Granulation Method
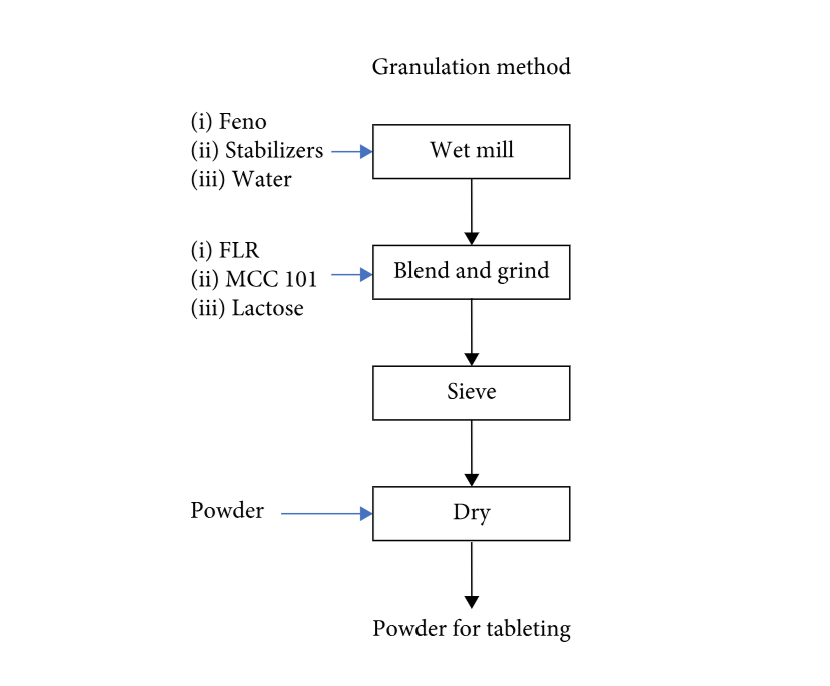
The pharmaceutical industry has highlighted particle-size reduction via preparing aqueous suspensions containing nano- or submicron drug particles. Owing to the risk of agglomeration and complications during the manufacturing of solid dosage forms, the problems associated with the solidification of nanosuspensions need to be addressed. Herein, the nanocrystallized suspension of fenofibrate (Feno) was prepared using the wet-milling technique, and then two solidification methods, mixing (liquid mixing) and granulation (dry powder blending and wet massing) methods, were investigated. The solidification process involved the adsorption of Feno as a very thin layer on the high-surface-area Florite® to prevent drug accumulation.
The critical quality attributes, particle size and dissolution rate, were performed. Infrared spectroscopy, X-ray diffraction, differential scanning calorimetry, and scanning electron microscopy were also used to monitor the existence and physical state of drug molecules in the carrier. The final solidified powders and tablets containing Feno nanocrystals improved the dissolution profile (>90% in 15 min), in which the physical properties of Feno were maintained during solidification and tableting. In general, the granulation method is more advantageous than the mixing method in terms of maintaining amorphous proportion and dissolution rate. These results implied a potential approach for manufacturing solid dosage forms from nanoscale products.
Download the research article as PDF: Solidifying Fenofibrate Nanocrystal Suspension
or read it here
Materials
Fenofibrate (Feno; 99.9%, USP43) was purchased from Oceanchinachem (Hubei, China). Florite-R®, FLR, was purchased from Tomita Pharmaceutical Co., Ltd. (Tokushima, Japan), and docusate sodium (DOSS) was purchased from Solvay SA (Belmont, WV, USA). Hydroxylpropyl methyl cellulose (HPMC E6) was provided by Colorcon (Indianapolis, PA, USA). Tribasic sodium phosphate, sodium lauryl sulfate (NaLS), potassium thiophosphate, and acetonitrile were purchased from Sigma–Aldrich (Merck, Darmstadt, Germany). Lactose monohydrate and sucrose were obtained from Evonik (Darmstadt, Germany). Microcrystalline cellulose (MCC 101; Mingtai Chemical, Bah-Der, Taiwan), croscarmellose sodium (Bionique Pharma, Surat, India), crospovidone CL (Polyplasdon CL, Ashland, Covington, KY, USA), and talc and magnesium stearate (Aurora Industry, Liaoning, China) were used without modification.
Bao Ngoc Tran, Hiep Tuan Tran, Giang Thi Le, Ha Phuong Tran, Khanh Ngoc Le, Huy Hoang Do, Anh Hoang Dao, Chien Ngoc Nguyen, “Solidifying Fenofibrate Nanocrystal Suspension: A Scalable Approach via Granulation Method”, Journal of Nanomaterials, vol. 2023, Article ID 1672030, 11 pages, 2023. https://doi.org/10.1155/2023/1672030

