Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization
Abstract
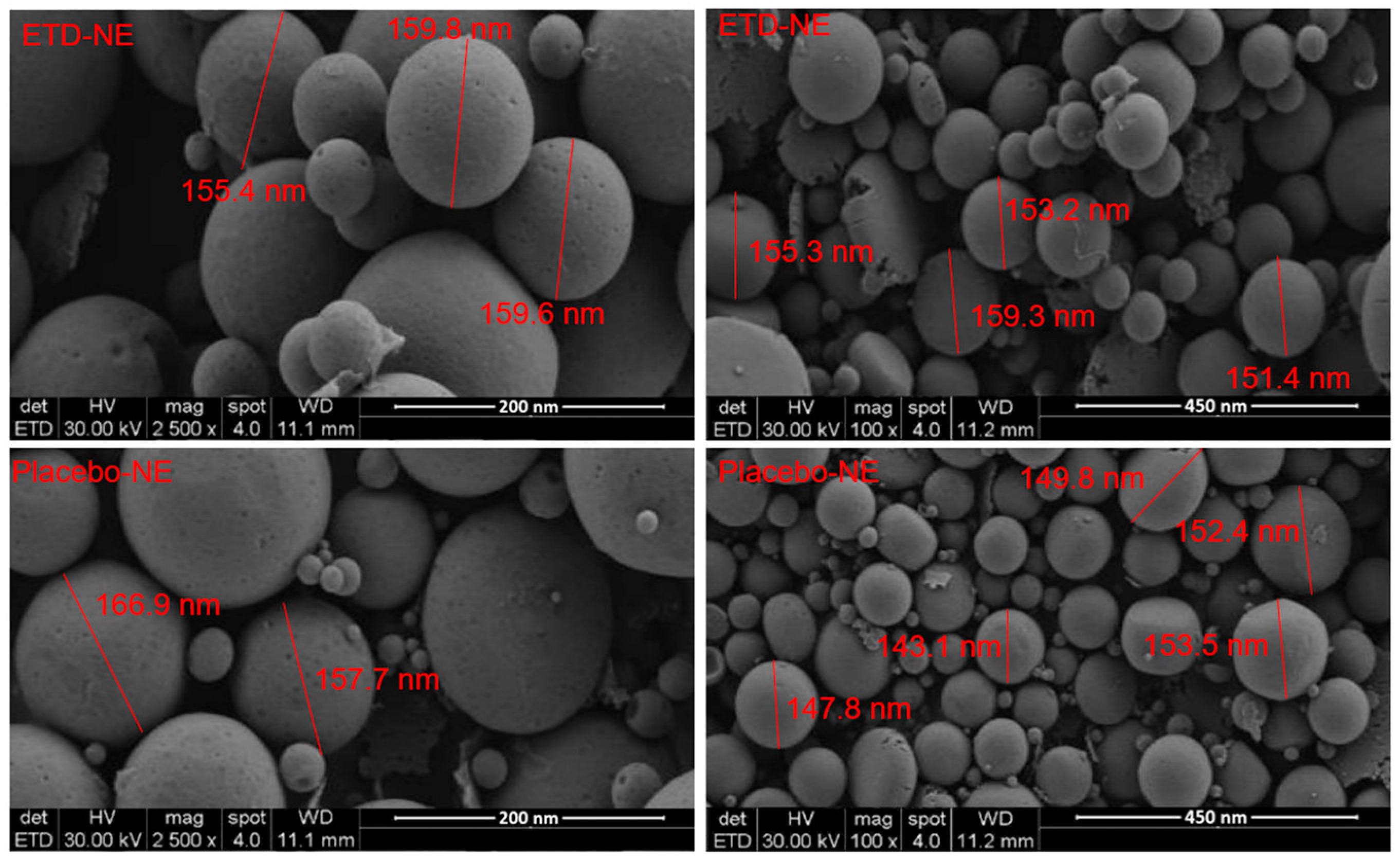
This research primarily focuses on the development of innovative topical nanoemulsions for etodolac, aimed at surmounting its inherent limitations. The preparation of etodolac nanoemulsions is accomplished through a combination of high shear homogenization and ultrasonication methods. The optimization of the formulation components is systematically conducted using the design of experiments methodology. The droplet size (DS), polydispersity index (PDI), and zeta potential (ZP) of the optimized formulation were assessed using the differential light scattering (DLS) technique. Surface morphology examinations were conducted using electron microscopy, while interactions between excipients and the drug were analyzed through FTIR analysis. Additionally, in vitro release and ex vivo permeability studies were carried out. Furthermore, anti-inflammatory activity was evaluated in the context of a carrageenan-induced paw edema model in rats. The DS, PDI, and ZP of the optimal formulation were 163.5 nm, 0.141, and −33.1 mV, respectively. The in vitro release profile was assessed as a sustained release by following a non-Fickian drug transport. The flux of etodolac nanoemulsions and coarse dispersions were 165.7 ± 11.7 µg/cm2 h and 59.7 ± 15.2 µg/cm2 h, respectively. Enhanced edema inhibition was observed at 13.4%, 36.5%, and 50.65% for the 6th, 8th, and 24th hours, respectively. Taken together, these results confirmed that nanoemulsions are promising carriers for the topical delivery of etodolac.
Introduction
Topical drug delivery has emerged as a viable alternative to conventional oral and parenteral routes, offering numerous advantages such as a localized treatment, reduced systemic side effects, a prolonged therapeutic effect, and improved patient compliance [1]. Despite these benefits, the Stratum corneum, the outermost layer of the skin, presents a formidable barrier for the drug permeation [2].The physicochemical properties (e.g., high melting points, hydrophilicity, or large molecular sizes) of drugs can limit their suitability for topical delivery. The main objective of extensive research is to overcome these limitations and improve the delivery of drugs through the skin. This drives the investigation of new formulations and delivery methods.
In this context, nanoemulsions have attracted significant attention in topical drug delivery. They provide a versatile and efficient platform for improving skin penetration and precisely releasing therapeutic agents. These innovative delivery systems, made of tiny oil or water droplets stabilized by surfactants or co-surfactants, showcase outstanding physicochemical properties suitable for topical use [3]. The small droplet size of nanoemulsions provides a large interfacial area, facilitating improved solubilization and dissolution rates of hydrophobic drugs, such as those with a limited aqueous solubility [3,4]. This characteristic enables the efficient incorporation and permeation of lipophilic drugs through the skin’s lipid barrier, enhancing their bioavailability and therapeutic efficacy. Hence, utilizing nanoemulsions for topical drug delivery holds great promise in overcoming the challenges associated with conventional drug delivery methods.
An example of a drug facing topical delivery challenges is etodolac, a nonsteroidal anti-inflammatory drug (NSAID) known for its strong analgesic and anti-inflammatory effects in the treatment of rheumatoid arthritis [5]. It is classified as a biopharmaceutical classification system (BCS) class II drug that is characterized by a poor aqueous solubility and high permeability [6,7]; however, its poor aqueous solubility and the formidable barrier posed by the skin hinder its effective topical permeation. Due to the relatively short duration of action in the body, oral administration of the medication requires frequent dosing; however, this increased dosing frequency can lead to adverse cardiovascular effects, fluid retention, edema, and gastrointestinal problems, including bleeding, ulceration, and perforation [7,8,9]. Recently, there has been a growing interest to overcome these challenges, and local or topical formulation strategies utilizing nano-sized systems have gained significant attention [6,7,8,9,10]. By encapsulating etodolac within a nanoemulsion system, it is possible to enhance its solubility, increase its penetration through the skin, and ultimately improve its therapeutic efficacy. This innovative approach showcases the potential of nanoemulsion-based drug delivery systems to address the limitations of conventional drug delivery, ultimately improving patient outcomes.
Numerous parameters play a significant role in the design of nanoemulsions considered as an alternative to the conventional dosage form of etodolac. Designing nanoemulsions requires the precise selection of ingredients to achieve effective drug incorporation, targeting, and desired release patterns. It is crucial to optimize factors like the ratio of oil to water, the ratio of surfactant to co-surfactant, and the techniques used for mixing to attain a stable and efficient nanoemulsion formulation [11]. To streamline this process, the design of experiment (DOE) approach offers a systematic and efficient methodology. By employing DOE, multiple factors and their interactions can be assessed simultaneously, leading to a better understanding of the formulation’s critical quality attributes (CQAs) [12]. In this approach, various factors such as surfactant type and concentration, oil phase composition, and processing conditions are carefully selected and varied according to a predefined experimental design matrix. By systematically studying the effects of these factors on the response variables of interest, such as droplet size, polydispersity, and physical stability, an optimized formulation can be identified. Statistical analysis of the experimental results helps identify significant factors and their interactions, enabling the formulation scientist to make data-driven decisions and fine-tune the nanoemulsion formulation for the desired properties. The DOE approach significantly reduces the number of experiments required and provides valuable insights for formulation optimization, leading to the development of stable and efficient nanoemulsions of etodolac [11].
The present investigation aims to formulate an optimal nanoemulsion of etodolac to enhance its skin penetration. Key characteristics of the formulation and process parameters were monitored, then the DOE approach was employed to optimize the nanoemulsion formulation. The interaction between the formulation variables was investigated and the droplet size, droplet size distribution, zeta potential, and encapsulation efficiencies were evaluated as the response variables. After physicochemical characterization studies of the optimal nanoemulsion formulation in vitro, ex vivo and in vivo studies were performed.
Download the full article as PDF here Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization
or read it here
Materials
The etodolac was kindly provided by Nobel Pharmaceuticals (Istanbul, Turkey). Caprylic/capric triglyceride (Crodamol™ GTCC), isopropyl isostearate (Crodamol™ IPIS), isopropyl myristate (Crodamol™ IPM) and isopropyl palmitate (Crodamol™ IPP) were obtained from Croda Turkey (Istanbul, Turkey). Castor oil, olive oil, sesame oil, Poloxamer® 188 and Poloxamer® 407, Brij® 35 and tyloxapol were supplied by Sigma-Aldrich (Schnelldorf, Germany). Tego Care® 450 was provided by Evonik Industries (Essen, Germany). All the chemicals and reagents used in the formulation, production, and analysis stages were of analytical grade.
Özdemir, S.; Üner, B.; Karaküçük, A.; Çelik, B.; Sümer, E.; Taş, Ç. Nanoemulsions as a Promising Carrier for Topical Delivery of Etodolac: Formulation Development and Characterization. Pharmaceutics 2023, 15, 2510. https://doi.org/10.3390/pharmaceutics15102510

