Comparison of properties on Orally Disintegrating Tablets (ODTs) produced by direct compression or fluid bed granulation

Abstract
Orally disintegrating tablets (ODTs) is a popular drug delivery system as they dissolve in the mouth, usually within seconds which enables easy medication for patients with problem swallowing. In this thesis properties of ODTs were compared when produced with direct compression of the excipients or the excipients were pretreated to form granules in a fluid bed granulator prior to tableting.
By altering the excipients used and the process parameters during fluid bed granulation it was concluded that the choice of polyol as the main filler in the tablet mostly affected the properties of both the granules and the produced tablets. This was also concluded for the tablets produced with direct compression. The granulation process increased the flowability of the particles.
The disintegration time was also mostly affected by the filler and not the used super disintegrants. Tablets containing mannitol disintegrate faster than tablets with isomalt or xylitol and is also less friable. From the measured in vivo disintegration times an in vitro method was developed with the aim to give a better in vitro-in vivo correlation (IVIVC) compared to existing methods. The developed method is applicable for tablets containing mannitol as they swell when disintegrating. For this a texture analyzer was used which applied and measured the force on tablets when swelling.
2.4 Excipients
2.4.1 Fillers
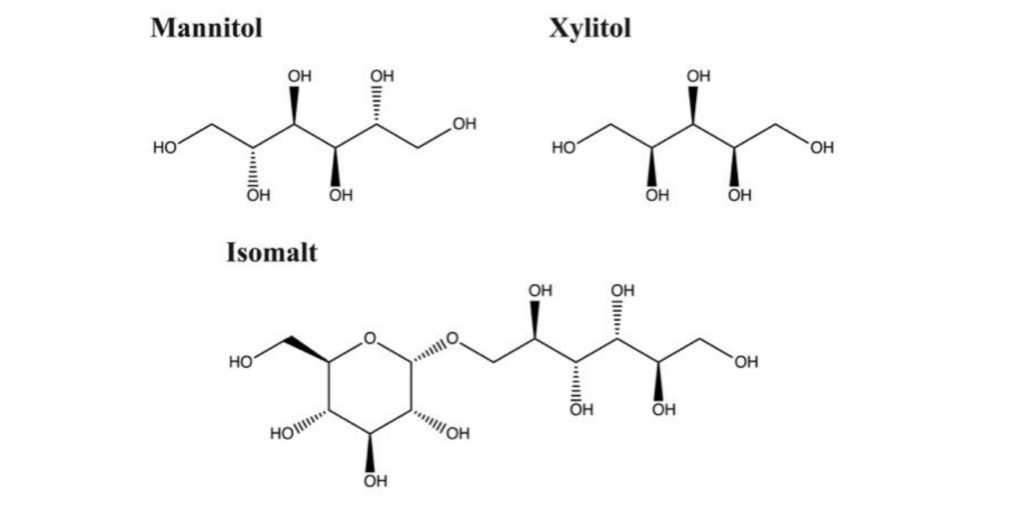
Polyols can be used as fillers to dilute the concentration of the API in the tablet and to improve the taste of the ODT since they are sweet and inert towards both the API and the body. Mannitol, isomalt and xylitol (Fig. 2.2) are polyols with different properties readily used in the pharma industry for producing solid tablets (Mitchell, 2006). Xylitol is the sweetest of the three polyols, almost as sweet as sugar while mannitol and isomalt gives about 45-70 % of the sweetness from sugar. (Lenhart and Chey, 2017). Xylitol is also the most soluble, about 67 % (w/w) while mannitol is the least soluble of the three, about 20 % (w/w) and isomalt has a solubility of 25 % (w/w) (Mitchell, 2006). A more soluble excipient makes the granules more uniform in particle size and decreases the friability (Hiremath, Nuguru and Agrahari, 2019). Both mannitol and isomalt are non-hygroscopic which is an advantage when storing the produced tablets since they have a low water absorbance tendency. Again, xylitol differs from the other two and is relatively hygroscopic (Mitchell, 2006). Both mannitol, isomalt and xylitol shows good compressibility which makes them suitable for production of solid tablets (Bin et al., 2020), (Lura et al., 2019).
Another type of molecule acting as both filler, binder and disintegrant is microcrystalline cellulose (MCC) (Roquette, 2023). MCC may increase the flow properties of the granules (Yassin et al., 2015). On the contrary to mannitol and isomalt, MCC is highly hygroscopic which enables it to absorb moisture (Hiremath, Nuguru and Agrahari, 2019). MCC, on the contrary to the polyols, is insoluble in water (FAO, 1997).
2.4.2 Binders
Binders enhance the binding between the molecules and facilitates formation of larger agglomerates. The binder can be added as a powder or in liquid form, however the bonds are formed by establishing a wet surface on the particles and make them agglomerate into larger particles facilitated by the binder. Altering the binder may affect the size of the granule since they can bind them with different strength (Dürig and Karan, 2019).
A commonly used binder is pregelatinized starch which is produced by rupturing the native structure of starch. The modifications increase solubility, flowability and compressibility compared to the native starch and makes it a suitable binder, however it can also act as a disintegrant (Dürig and Karan, 2019). When partially pregelatinized the starch receive both soluble and insoluble properties. Pregelatinized starch is available as Lycatab PGS (fully pregelatinized) from Roquette and Starch 1500 (partially pregelatinized) from Colorcon.
2.4.3 Lubricants
Lubricants are added to the formulation before tableting to enhance the flowability and decrease the friction on the equipment during tableting by coating the particles. A commonly used lubricant is magnesium stearate which consists of a charged magnesium molecule interacting with the powder particles or granules and two fatty acids of stearic or palmitic acid which locates away from the particle and form a hydrophobic surface which gives the particle glidant properties (Hiremath, Nuguru and Agrahari, 2019).
2.4.4 Disintegrants
Disintegrants or super disintegrants ensure that the ODT disintegrate rapidly once in contact with liquid. There are mainly four different mechanisms by which the disintegrants act to make the tablet dissolve, called wicking, swelling, elastic recovery and repulsion. The first disintegrant used was starch which is still used in different modified forms as both disintegrant and super disintegrant (Desai, Liew and Heng, 2016). Starch can act as a disintegrant if partially pregelatinized, if fully pregelatinized it will dissolve in water and is unable to act as a disintegrant. When partially pregelatinized the starch have both soluble properties from the gelatinization process and insoluble properties from the native starch, the insoluble properties ensuring that disintegration is possible (Hiremath, Nuguru and Agrahari, 2019).
Today, two of the most used disintegrants are croscarmellose sodium (CCS) and sodium starch glycolate (SSG) (Markl and Zeitler, 2017), both are synthetic polymers of modified cellulose and starch respectively (Berardi, Janssen and Dickhoff, 2022). Both CCS and SSG are modified to reduce the solubility and viscosity compared to the native molecule. This results in controlled swelling of the disintegrant in contact with water (Berardi, Janssen and Dickhoff, 2022). SSG acts mainly by swelling, it has a hydrophilic carboxymethyl group which is embedded in a hydrophobic phosphate ester, and in contact with water it swells and breaks the intermolecular bonds of the granules (Dilebo and Gabriel, 2019). CCS have less swelling capacity than SSG due to that the structure of cellulose is more linear while the
starch structure of SSG is more branched. This allows less space for water to enter the complex of CCS and thus the swelling decreases (Berardi, Janssen and Dickhoff, 2022). Instead, the dominating disintegration mechanism for CCS is wicking. By wicking, capillary forces draw water inside the tablet where the water disrupts interparticular bonds and dissolve the tablet (Sabath, 2023).
Crospovidone is another typical disintegrant which is hydrophilic, however with poor solubility in water and low viscosity. Due to the lower viscosity and solubility the disintegrant acts mainly by wicking. A lower viscosity enables easier water penetration of the tablet and a faster disintegration is possible (Berardi, Janssen and Dickhoff, 2022). Crospovidone may also act by swelling and elastic recovery (Sabath, 2023), although the swelling capacity in crospovidone is limited (Berardi, Janssen and Dickhoff, 2022). The swelling effect is limited for soluble particles and for highly porous particles containing void spaces (Zhou et al., 2014). Elastic recovery or shape recovery is when the particles can regain their original shape they had before compression into tablets, once exposed to liquid. When changing shape stored energy is released and the tablet disintegrate (Berardi et al., 2021).
3.1 Materials
The raw material of mannitol (Pearlitol 150 SD), MCC (Microcel-101), croscarmellose sodium (Solutab A), xylitol (Xylisorb XTAB 400) and starch (Lycatab PGS) was received from Roquette. Starch 1500 was received from Colorcon and isomalt (GalenIQ 721) was received from Beneo. Crospovidone (Vivapharm XL-10) and sodium starch glycolate (Vivastar P) was received from JRS Pharma.
Download the full Master Thesis by Julia Ahorn “Comparison of properties on Orally Disintegrating Tablets (ODTs) produced by direct compression or fluid bed granulation“ here:
(click the picture below)
Source: Julia Ahlbom, Master Thesis Project 2023 “Comparison of properties on Orally Disintegrating Tablets (ODTs) produced by direct compression or fluid bed granulation”
Read more on Orally Disintegrating Tablets (ODTs) here:



