An Investigation of a Novel, Directly Compressible, Oil-carrying Excipient in a Solid, Self-emulsifying Delivery System
JRS Pharma presented three posters at the 2024 PBP World Meeting in Vienna. This is the third poster:
Introduction
Currently in the small-molecule therapeutic arsenal around40%oftheactivepharmaceuticalingredients (APIs) are poorly water-soluble with up to 90 % of the APIs in current development pipelines belonging to the Biopharmaceutical Classification System (BCS) classes II (poor solubility, high permeability) and IV (poor solubility, poor permeability).
Within oral solid dosage forms (OSDFs), tablets are the preferred OSDF for both the manufacturer and the patient. However, formulating poorly water-soluble APIs (BCS classes II and IV) into tablets remains a challenge. Self-emulsifying Drug Delivery Systems (SMEDDS) are a well stablished strategy to formulate BCS class II and IV
actives and enhance their bioavailability, but they often [1,2] present limitations.
SMEDDS can be formulated as liquids or solids with limited studies reporting that in terms of bioavailability liquid
[3] SMEDDS are superior to solid SMEDDS. The oral absorption of said APIs depends on many formulation-related parameters, such as surfactant concentration, oil/surfactant ratio and droplet size . Excipients might play an important role in improving the bioavailability of BCS class II and IV APIs, not only by enabling and stabilising the
emulsion formation and diminishing droplet size at the target drug release, but also by facilitating the manufacturing process for both solid and liquid oral dosage forms; the formulation of a tablet based SMEDDS is therefore desirable.
Purpose of the Study
Creating a stable emulsion is a key factor to improve the bioavailability of BCS class II and IV APIs. In this study, different formulations based on PROSOLV® 730 (a coprocessed excipient comprising microcrystalline cellulose, silica and copovidone) were tableted as a proof of concept and foundational work towards a solid SMEDDS prototype formulation.
The study evaluates the emulsion formation and stabilisation capabilities of a formulation in which PROSOLV® 730 was loaded with an oil system (Miglyol 812) containing a model compound (Sudan Blue II – SBII) when formulated in a tablet in conjunction with Poloxamer 188.
Methods
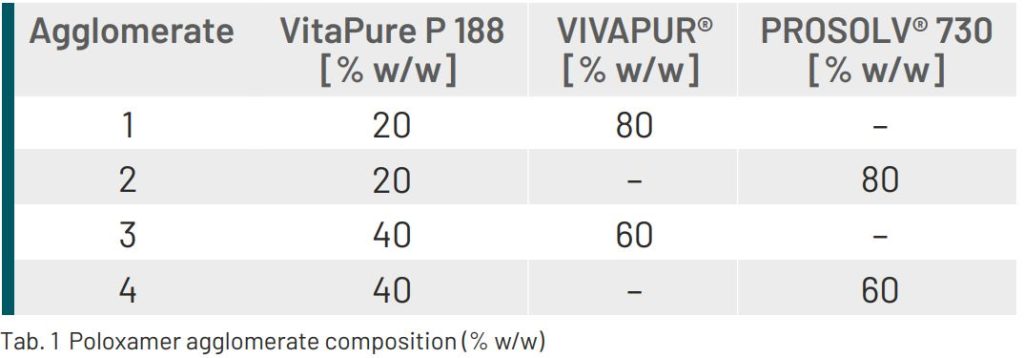
A poloxamer, VitaPure P 188 (Clariant Produkte GmbH, Germany), is a solid, waxy material with a melting point of
52 °C. With the aim of facilitating its handling and blending properties, VitaPure P 188 was melted in a
microwave oven and then agglomerated with either VIVAPUR® 101 (MCC) or with PROSOLV® 730 respectively at
2 different concentrations, resulting in 4 different co-processed materials.
VIVAPUR® 101 and PROSOLV® 730 were preheated at 70 °C before introducing them in the blender, which was heated at 80 °C. The blender was set to mid-speed (5/10) and the molten poloxamer was poured over the powder at a
constant rate for 3 minutes. The granulation process went on for 7 more minutes at 80 °C.
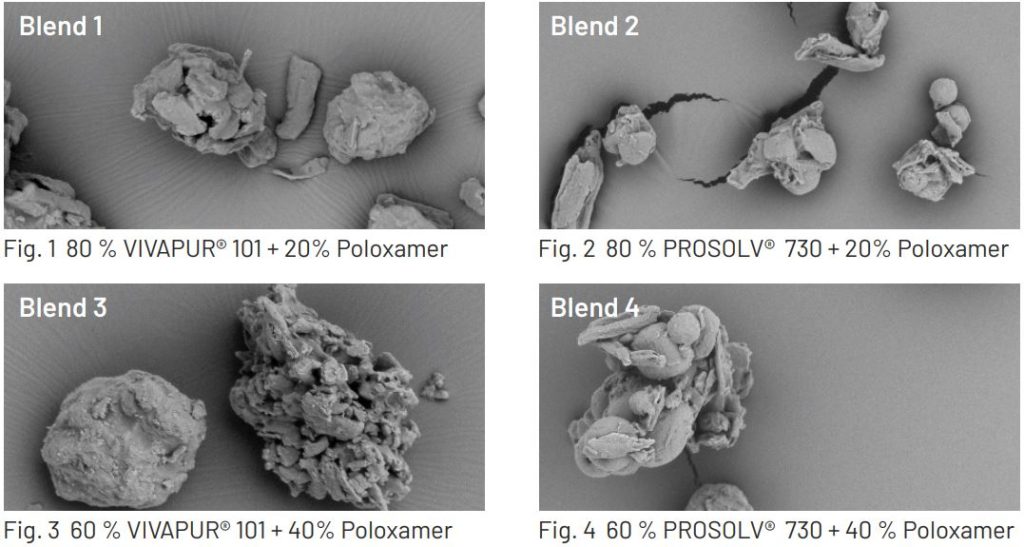
Obtained agglomerates were sieved over a 100 µm hand sieve. Images were obtained using a scanning electron
microscope (RT1000 HITACHI, Japan).
Blends were prepared of the SBII-loaded PROSOLV® 730 with the various agglomerates. The main components
were initially blended for 15 minutes using a free-fall blender (Brunimat, Switzerland), with 3 minutes of additional
blending after lubricant addition. The composition of the blends were as follows (Table 2):
![Tab. 2 Blend composition [% w/w]](https://www.pharmaexcipients.com/wp-content/uploads/2024/04/Tab.-2-Blend-composition-300x159.jpg)
For every blend, 5 tablets were dissolved in a beaker and mixed for 10 minutes in 100 mL of demineralized water
with the help of a high-performance disperser Ultra-Turrax T25 (IKA Werke GmbH & Co. KG; Germany). The
homogenised suspension was then poured in a 100 mL volumetric cylinder, and its appearance was photographed over the span of 1 week. This experiment was performed in duplicate for each blend.
Results
All of the agglomerates were successfully produced and screened. The SEM images confirmed successful agglomeration of the individual ingredients in the case of VIVAPUR® blends and possibly a combination of both loading/adsorption and agglomeration in the PROSOLV® 730 based blends. The blends flowed well in the tablet die and tablets were pressed with a hardness of 80-90 N. Smooth tablets with no imperfections or structural issues such as lamination or capping were produced. The homogenized suspensions were allowed to settle in volumetric cylinders and were visually inspected for stability.
A picture was taken at time-points 15 min, 60 min, 16 h, 24 h, 28 h and 1 week for every blend (Figures 5-9). After the insoluble ingredients settle down (during the first 24 hours), a bright blue non-transparent phase is observed, corresponding to an emulsion formed in the aqueous phase. A minor oil phase can be observed on the surface in the final days of the experiment. For Blends 5-8 the emulsion phase remained visually stable with minor to no changes in its color and aspect. The control blend (Blend 5) separated noticeably by the 16 h time-point.
Conclusion
Every formulation showed emulsion stability for 1 week after tablet disintegration when compared to the negative control. PROSOLV® 730 showed excellent carrying capacities for miglyol in every formulation and for the molten poloxamer in formulations 3 and 4, while maintaining good flow and tabletability.
Download the full poster on “Investigation of a Novel, DC, Oil-carrying Excipient” here
(click on the image to see them enlarged)
Source: JRS Pharma, PBP World Meeting, poster “Investigation of a Novel, DC, Oil-carrying Excipient“, Guillermo Sánchez-Villarta ; Anthony Carpanzano ; Gernot Warnke, JRS PHARMA GmbH & Co. KG, Holzmühle 1 Rosenberg, Germany, Guillermo.Sanchez[at]jrspharma.de, JRS PHARMA LP, 2981 Route 22 Patterson, NY, USA, Anthony.Carpanzan[at]jrspharma.com, JRS PHARMA GmbH & Co. KG, Holzmühle 1 Rosenberg, Germany, Gernot.Warnke[at]jrspharma.de
See the other two JRS posters from PBP World Meeting:
- Multi-Particulate Formulation of Pantoprazole
- Tabletability of Roll Compacted Binary Mixtures of Dicalciumphosphate with Silicified and Non-Silicified MCC
Do you need more information or a sample of JRS Pharma excipients?