Oxidative Stability in Lipid Formulations: a Review of the Mechanisms, Drivers, and Inhibitors of Oxidation
Abstract
The importance of lipid-based formulations in addressing solubility and ultimately the bioavailability issues of the emerging drug entities is undeniable. Yet, there is scarcity of literature on lipid excipient chemistry and performance, notably in relation to oxidative stability. While not all lipid excipients are prone to oxidation, those with sensitive moieties offer drug delivery solutions that outweigh the manageable oxidative challenges they may present. For example, caprylocaproyl polyoxylglycerides help solubilize and deliver cancer drug to patients, lauroyl polyoxylglycerides enhance the delivery of cholesterol lowering drug, and sesame/soybean oils are critical part of parenteral nutrition. Ironically, excipients with far greater oxidative propensity are omnipresent in pharmaceutical products, a testament to the manageability of oxidative challenges in drug development. Successful formulation development requires awareness of what, where, and how formulation stability may be impacted, and accordingly taking appropriate steps to circumvent or meet the challenges ahead. Aiming to fill the information gap from a drug delivery scientist perspective, this review discusses oxidation pathways, prooxidants, antioxidants, and their complex interplay, which can paradoxically take opposite directions depending on the drug delivery system.
Download the full research paper as PDF: Oxidative Stability in Lipid Formulations – A Review of the Mechanisms, Drivers, and Inhibitors of Oxidation
Introduction
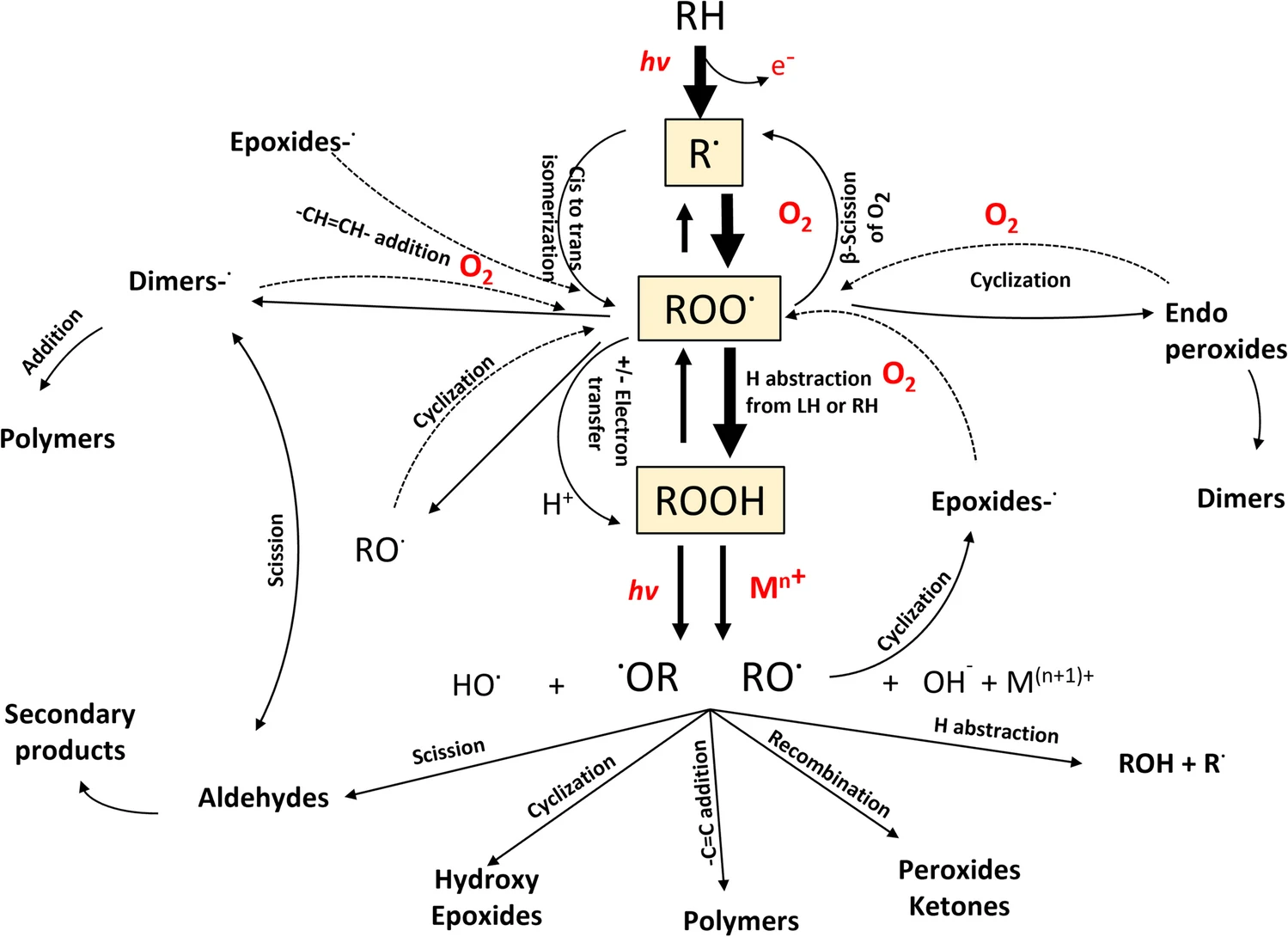
Lipid formulations vary by the excipients and the technologies used in their development. The simplest example of a lipid formulation is that of a poorly soluble drug dissolved in fixed oil(s). Fixed oils are monographed vegetable oils (e.g., soybean, sesame), with well-defined composition in triglycerides and fatty acid distribution. Self (micro)-emulsifying drug delivery systems (SEDDS, SMEDDS) on the other hand may consist of fixed oils, partial glycerides, polyoxylglycerides, polysorbates, and/or propylene glycol esters combined with a polyethylene glycol. Some preparations may be of liquid consistency, filled into capsules. Others may be developed as solid particles by dispersion, adsorption onto solid carriers, or melt granulation/congealing techniques. Solid lipid nanoparticles, microemulsions, and liposomal carriers should not be excluded from this non-exhaustive list of possibilities. Nevertheless, few publications offer pharmaceutically relevant and aggregated information on the oxidative stability of such complex lipid formulations.
Meanwhile, the core causes of oxidative instability in lipid-based formulations remain misunderstood due in part to the large number of parameters and antagonisms governing oxidative instability. As a result, the misperceptions that lipid-based formulations present a risk of failure during stress and stability testing continue to linger. There is no factual basis for this thinking because lipid formulations have for many decades been used in oral, topical, and parenteral applications. Ether-based polymers with far greater propensity to oxidation such as polyethylene glycols (macrogols) and polyoxyethylene glycol–polyoxypropylene glycol block copolymers (poloxamers) are commonly found in marketed drug products. At minimum, these achievements are testament to the manageability of oxidative events in pharmaceutical products.
Combing the literature, we find confusing reviews, exemplified by an article opining on lipids and SEDDS, citing them as complex and as carriers of reactive impurities. Yet, the examples of chemistries cited were solubilizers based on polyethylene oxide (PEO), polypropylene oxide (PPO), and polyethylene glycols (PEG). Also cited were the account of an “unstable” drug X in a SEDDS formulation of unidentified composition, or the peculiar case of drug Z reacting with its sole carrier, oleic acid — even though free fatty acids are known as strong prooxidants. Such anecdotal references emerge when fundamentals of formulation science are overlooked, and formulation attempts fail for the wrong reasons.
Undeniably, oxidative change may lead to loss of drug potency, diminished shelf life, and time to market. Incomplete assessment of oxidative risks may translate to missed opportunities in applying antioxidants or a wrong antioxidant system, leading to acceleration of undesirable reactions. However significant, oxidative challenges are not unsurmountable. Appropriate choices in excipient selection, formulation/process, and antioxidant selection are key to successful development. Apprised knowledge about oxidative pathways becomes an essential part of a new thinking about lipid-based drug delivery systems. The aim of this paper is to provide a consolidated review of the current understanding of oxidative events encountered in lipid formulations; to underline oxidative risks, where they lie; and offer insight on how they can be prevented or managed.
Musakhanian, J., Rodier, JD. & Dave, M. Oxidative Stability in Lipid Formulations: a Review of the Mechanisms, Drivers, and Inhibitors of Oxidation. AAPS PharmSciTech 23, 151 (2022). https://doi.org/10.1208/s12249-022-02282-0

