Novel inhalable powder formulation of pirfenidone with sustained release properties to improve pulmonary deposition
Abstract
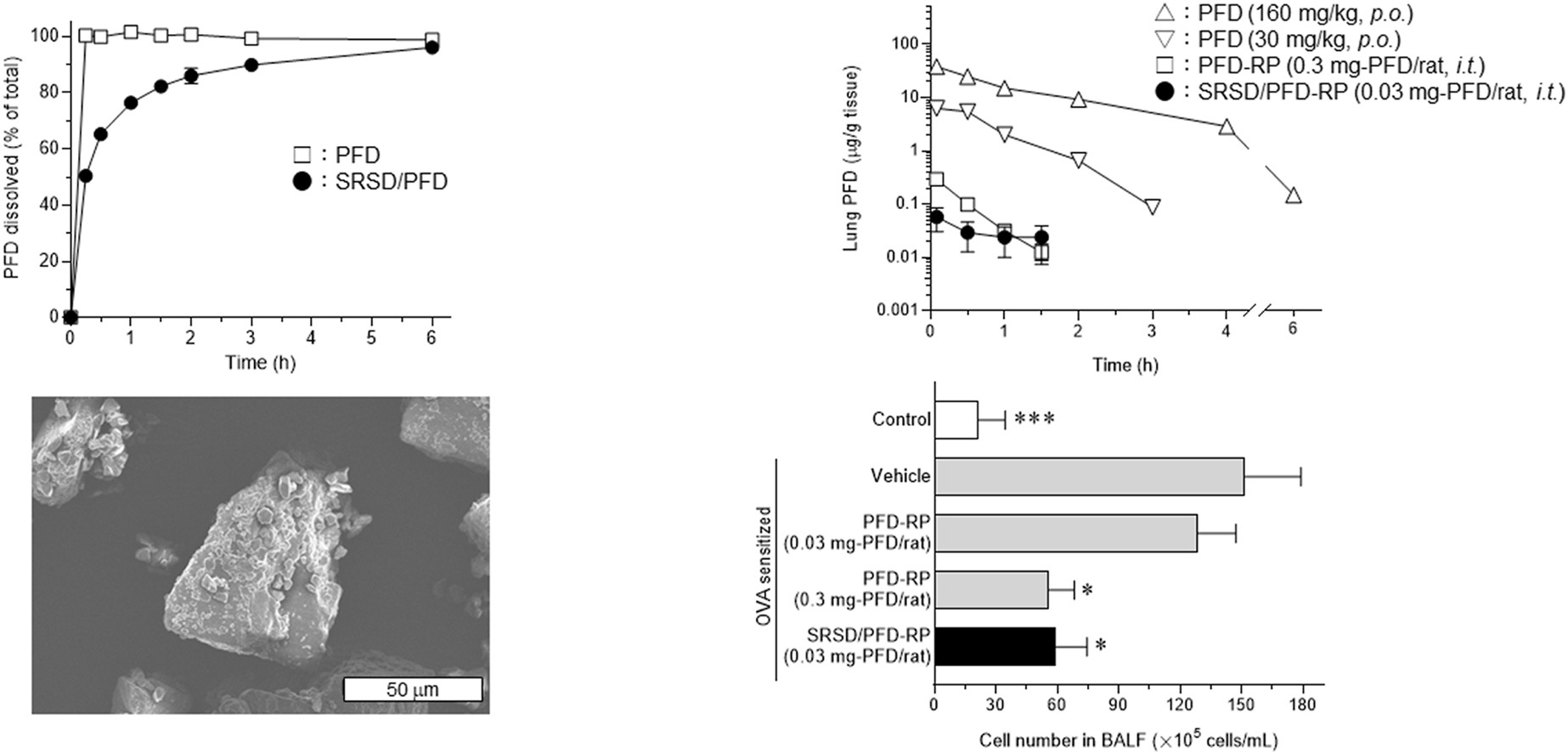
The aim of this study was to develop a new respirable powder (RP) formulation of pirfenidone (PFD) with sustained release properties to ameliorate the pharmacokinetic drawbacks of the previously-developed PFD-RP on rapid elimination from the lung after insufflation. Based on screenings using several polymers, Kollidon® SR was chosen to produce a solid dispersion of PFD with sustained release properties (SRSD/PFD), and an RP formulation of SRSD/PFD (SRSD/PFD-RP) was prepared. Characteristics of SRSD/PFD-RP were examined in terms of biopharmaceutical, pharmacological, and phototoxic properties. In laser diffraction analysis, SRSD/PFD-RP could be dispersed to SRSD/PFD and lactose carrier particles, and SRSD/PFD in SRSD/PFD-RP had suitable particle sizes for inhalation. Insufflated SRSD/PFD-RP (0.03 mg-PFD/rat) showed a prolonged elimination half-life and mean residence time of PFD since these values were approximately 3.7-fold higher than those of insufflated PFD-RP (0.3 mg-PFD/rat: a pharmacologically-effective dose). Insufflated SRSD/PFD-RP (0.03 mg-PFD/rat) showed a similar anti-inflammatory potential in the lung of antigen-evoked lung inflammatory models compared with insufflated PFD-RP (0.3 mg-PFD/rat). Obvious skin phototoxicity was negligible after insufflation of SRSD/PFD-RP (0.03 mg-PFD/rat). In conclusion, SRSD/PFD-RP would be an attractive dosage form for an inhalation system of PFD and contribute to PFD medication for idiopathic pulmonary fibrosis with high efficacy and safety.
Highlights
- A new respirable powder formulation of pirfenidone (PFD-RP) was developed.
- Solid dispersion of PFD with sustained release properties (SRSD/PFD) was applied.
- Insufflated SRSD/PFD-RP (0.03 mg-PFD/rat) showed sustained pulmonary deposition.
- Insufflated SRSD/PFD-RP showed favorable anti-inflammatory potential in the lung.
- SRSD/PFD-RP would contribute to efficacious PFD medication by inhalation system.
Introduction
Pirfenidone (PFD) is used as an oral agent to treat idiopathic pulmonary fibrosis (IPF) [[1], [2], [3]]. PFD is known to have anti-fibrotic and anti-inflammatory potential via the regulation and inhibition of various inflammatory and fibrotic pathways [[4], [5], [6], [7], [8], [9]]. Although these beneficial effects for IPF patients, severe and frequent side effects have been reported, such as digestive symptoms and phototoxic dermatitis after oral administration [1,10,11]. The occurrence of these side effects led to a reduction in dosage or discontinuation of PFD in some patients with IPF [11,12]. Therefore, to achieve efficacious PFD treatment with wide safety margins, the avoidance or reduction of the risk of orally-dosed PFD-induced adverse effects is urgently required. Possible onset mechanisms of the adverse effects induced by orally-taken PFD have been reported [13,14], and exposure of the digestive tracts and skin to PFD may be associated with the occurrence of gastrointestinal discomfort and phototoxic skin responses, respectively. In this context, reduction of exposure of non-targeted tissues to PFD may reduce the risk of PFD-induced adverse effects.
To achieve efficacious and safe PFD medication for IPF patients, inhalation systems have been investigated [[15], [16], [17], [18], [19]], and respirable powder formulation of PFD (PFD-RP) has been newly developed to maximize topical efficacy of PFD for the lung and minimize the risk of side effects [20]. Insufflation of PFD-RP (0.3 mg-PFD/rat) could attenuate antigen-evoked lung inflammatory responses and achieve markedly lower systemic exposure to PFD compared with orally-dosed PFD at toxic doses [20,21]. Indeed, intestinal motility attenuation and dermal phototoxic events could not be observed after intratracheal administration of PFD-RP at the pharmacologically-effective dose because of limited exposure of the gastrointestinal tract and skin to PFD. On the other hand, a pharmacokinetic drawback of insufflated PFD-RP was identified in a previous investigation, specifically its rapid elimination from the lung, characterized by an elimination half-life of approximately 0.28 h [20]. Rapid elimination from the lung may necessitate frequent dosing of PFD-RP for IPF patients to achieve sufficient pharmacological efficacy; therefore, pharmacokinetic remediation to prolong retention of PFD in the lung would be required to treat IPF by inhalation systems of PFD. Sustained release techniques have been used to control drug absorption and the plasma concentration [22,23], and sustained release formulations contribute to the duration of efficacy and reduction of the risk of side effects [24,25]. There are limited reports on pharmacokinetic remediation of inhalation systems of PFD using sustained release techniques.
The aim of this study was to develop a new RP system of PFD with sustained release properties to ameliorate the pharmacokinetic drawback, particularly the rapid elimination of PFD from the lung, observed in the previously-developed PFD-RP. Several polymers were employed to fabricate solid dispersion of PFD (SD/PFD), and the dissolution behavior of SD/PFDs was examined. Based on the results of dissolution tests of SD/PFDs, SD/PFD with sustained release properties (SRSD/PFD) was chosen to prepare an RP formulation of SRSD/PFD (SRSD/PFD-RP), and physicochemical characterizations of SRSD/PFD-RP were undertaken to assess crystallinity, particle size, and surface morphology. The anti-inflammatory potential, pharmacokinetic characteristics, and in vivo skin phototoxicity after intratracheal administration of SRSD/PFD-RP were examined.
Read more here
Materials
PFD was bought from Tokyo Chemical Industry (Tokyo, Japan). Kollidon® 30, Kollidon 90 F , Kollidon® SR, and Eudragit® E PO were supplied by BASF Japan Ltd. (Tokyo, Japan). Pullulan was provided by Hayashibara Co., Ltd. (Okayama, Japan). Respitose® SV-003 and erythritol were supplied by DFE Pharma (Goch, Germany) and Nikken Chemicals (Tokyo, Japan), respectively. Aluminium hydroxide gel (Alhydrogel® 2%), horseradish peroxidase, and ovalbumin (OVA) were bought from Sigma-Aldrich.
Yoshiki Seto, Gen Suzuki, Masashi Kato, Hideyuki Sato, Satomi Onoue, Novel inhalable powder formulation of pirfenidone with sustained release properties to improve pulmonary deposition, Journal of Drug Delivery Science and Technology, Volume 94, 2024, 105487, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105487.
Read more articles on Pirfenidone here:
- Transdermal delivery and exploration of preclinical anti-rheumatoid efficacy of pirfenidone embedded nanoemulgel in adjuvant-induced rat model
- Fixed-dose dry powder for inhalation of nintedanib, pirfenidone and mycophenolic acid by thin-film freezing (TFF) technology
- Systematic Development and Optimization of Inhalable Pirfenidone Liposomes for Non-Small Cell Lung Cancer Treatment