Transdermal delivery and exploration of preclinical anti-rheumatoid efficacy of pirfenidone embedded nanoemulgel in adjuvant-induced rat model
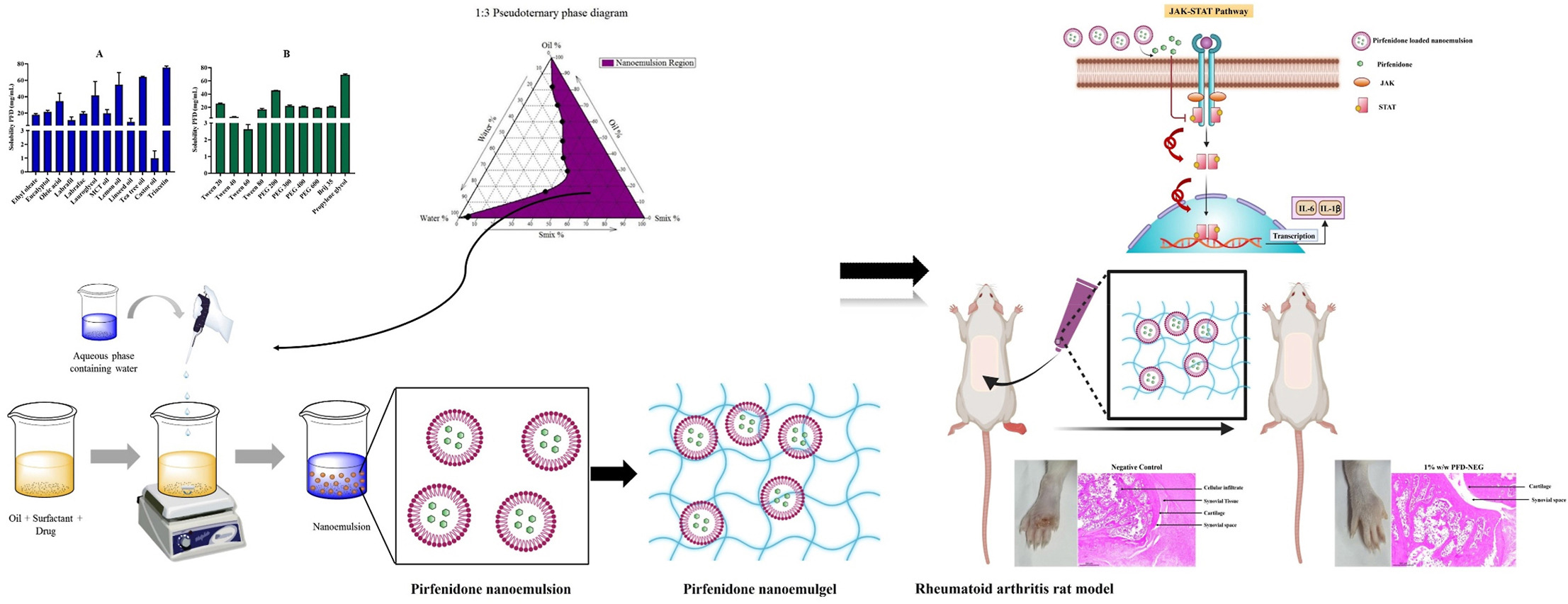
To avoid adverse effects associated with non-site-specific delivery and target major signaling pathways responsible for the inflammation in rheumatoid arthritis, the current study aims to formulate and evaluate the transdermally administered potential of pirfenidone nanoemulgel in rheumatoid arthritis. The pirfenidone was initially loaded into nanoemulsion by spontaneous nanoemulsification method. The optimized pirfenidone-loaded nanoemulsion diluted with distilled water has shown a droplet size of 9.46 ± 0.01 nm with uniform size distribution evaluated by photon correlation spectroscopy. The optimized pirfenidone nanoemulsion was converted into gel formulation by adding SEPINEO™ P600 gel base with the help of magnetic stirring.
The optimized pirfenidone-loaded nanoemulgel has shown a viscosity of 769 ± 05 Pa s with an antigravity nature. Ex vivo permeation study of pirfenidone nanoemulgel in BALB/c mice skin resulted in 1.7 times higher permeation of pirfenidone than free pirfenidone loaded gel. The preclinical efficacy of pirfenidone-loaded nanoemulgel has shown promising results in the rheumatoid arthritis-induced rat model. The right hind paw thickness (##p < 0.01) and arthritis severity score (####p < 0.0001) in pirfenidone nanoemulgel treated group resulted in significant reduction than negative control on 25th day of study. The spleen (****p < 0.0001) and liver indices (**p < 0.01) in rat group treated with pirfenidone nanoemulgel were found to be significantly reduced than negative control.
The serum levels of IL-1β (***p < 0.001) and IL-6 (**p < 0.01) were significantly decreased in the pirfenidone nanoemulgel treated group compared to negative control evaluated using enzyme linked immunosorbent assay. The radiographic images of pirfenidone nanoemulgel treated group showed reduction in swelling of soft tissue and bone erosion than negative control. The examination of the hind paw showed improved condition of paw joint with increased synovial space, reduction in the cellular infiltrates and decrease in the swelling of the joints in the pirfenidone nanoemulgel treated group.
Read more here
Materials
PFD was obtained as a kind gift from Cipla Pharmaceutical Ltd, Mumbai. Tween 40, Tween 20, Tween 80, Tween 60, Carbopol 934, Propylene glycol, Lemon oil, Castor oil. Eucalyptol, and Tea tree oil were purchased from Sisco Research Laboratories Pvt Ltd (SRL). PEG 300, PEG 200, PEG 600, PEG 400, Brij 35, Ethyl oleate, oleic acid and Triacetin oil were procured from Tokyo Chemicals (TCI). Labrafil, Labrafac and Lauroglycol and medium chain triglyceride (MCT) were obtained as a gift sample from Gattefossé.
Rimsha Nooreen, Shweta Nene, Ganesh Vambhurkar, Saurabh Srivastava, Transdermal delivery and exploration of preclinical anti-rheumatoid efficacy of pirfenidone embedded nanoemulgel in adjuvant-induced rat model, Journal of Drug Delivery Science and Technology, 2024, 105428, ISSN 1773-2247, https://doi.org/10.1016/j.jddst.2024.105428.
Read more articles on Transdermal Delivery here:
- Development of nanoemulsion loaded acyclovir nanogel for transdermal delivery and its evaluation
- Preparation, optimization and evaluation of Osthole transdermal therapeutic system
- Novel Therapeutic Hybrid Systems Using Hydrogels and Nanotechnology