Effect of roll compaction pressure on the properties of high drug-loaded piracetam granules and tablets
Objective
The aim of this study was to use an alternative granulation technique, solventless roll compaction, and to investigate the effect of the roll compaction pressure on the properties of granules and high-drug-loaded (80%, w/w) immediate release piracetam tablets.
Significance
Piracetam is commonly manufactured as high drug-loaded tablets by wet granulation with an aqueous binder solution. Due to its high solubility in water, the wet granulation process is largely susceptible to processing methods and can induce the uncontrolled polymorphic transition of piracetam as well as convert it into mono- and di-hydrates.
Methods
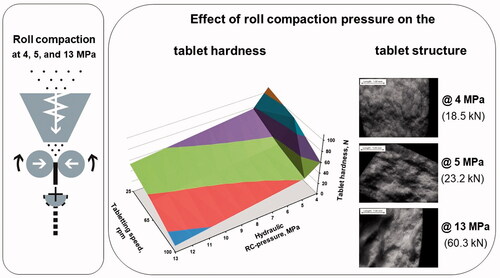
The blends, comprising piracetam, Kollidon® 30, and Avicel® PH-101 were roll compacted at 4, 5 and 13 MPa hydraulic pressure and calibrated using an industrial roll compactor. The resultant granules milled and raw piracetam was investigated with DSC. The resultant granules are mixed with Ac-Di-Sol®, Aerosil® 200 Pharma, and magnesium stearate to prepare tablets using an industrial tablet press at the same compression force and 25, 65, and 100 rpm. The obtained tablets were film coated with an aqueous dispersion of Opadry® II using a pilot-scale solid-wall pan coater.
Results
Roll compaction pressure influenced the polymorphic composition of piracetam, the granule properties and tablet mixture in relation to morphology, particle size, flowability, bulk and tapped density, as well as tablet hardness, tablet friability, disintegration, and dissolution.
Conclusion
This study showed that roll compaction can be successfully used for the preparation of highly water-soluble, highly drug-loaded piracetam film-coated tablets avoiding wet granulation pitfalls.
Download the research paper as PDF: Effect of roll compaction pressure on the properties of high drug-loaded piracetam granules and tablets
Read more
Materials
(2022) Effect of roll compaction pressure on the properties of high drug-loaded piracetam granules and tablets, Drug Development and Industrial Pharmacy,
DOI: 10.1080/03639045.2022.2123499

