Self-emulsifying drug delivery systems (SEDDS): In vivo-proof of concept for oral delivery of insulin glargine
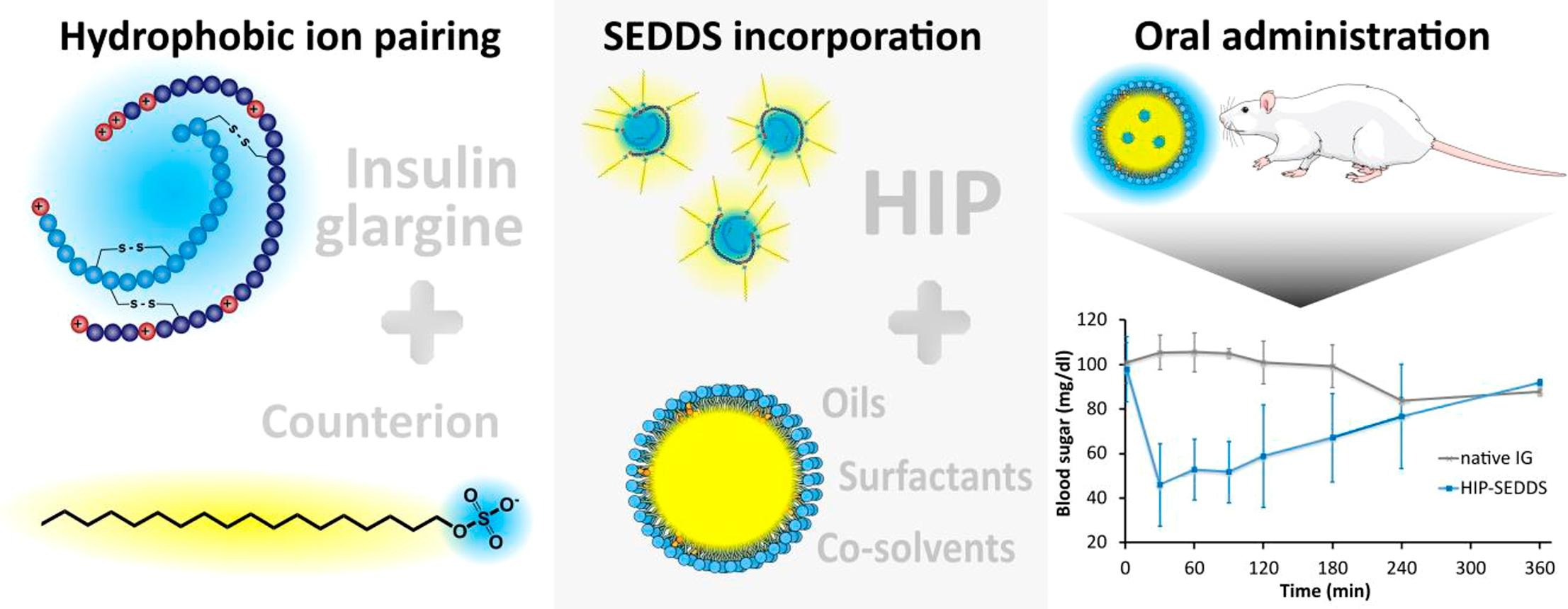
In spite of recent progress made in the field of peptide and protein delivery, oral administration of insulin and similar drugs remains a challenge. In this study, lipophilicity of insulin glargine (IG) was successfully increased via hydrophobic ion pairing (HIP) with sodium octadecyl sulfate to enable incorporation into self-emulsifying drug delivery systems (SEDDS). Two SEDDS formulations (F1: 20% Labrasol® ALF, 30% polysorbate 80, 10% Croduret 50, 20% oleyl alcohol, 20% Maisine® CC; F2: 30% Labrasol® ALF, 20% polysorbate 80, 30% Kolliphor® HS 15, 20% Plurol® oleique CC 497) were developed and loaded with the IG-HIP complex.
Highlights
- Hydrophobic ion pairs formed between insulin glargine and sodium octadecyl sulfate provided the highest increase in lipophilicity amongst investigated counterions.
- Two SEDDS formulations (F1 and F2) were developed, resulting in a sufficient solubility of 7.7 ± 2.5 and 6.4 ± 2.4 mg/ml of the hydrophobic ion pair, respectively.
- Log DSEDDS/release medium > 2 were achieved for both formulations.
- Formulations containing the ion-paired insulin glargine were administered via oral gavage to rats and resulted in a 7.7-fold (F1) and 6.2-fold (F2) increased bioavailability compared to an aqueous IG solution.
Further experiments confirmed increased lipophilicity of the complex, achieving LogDSEDDS/release medium values of 2.5 (F1) and 2.4 (F2) and ensuring sufficient amounts of IG within the droplets after dilution. Toxicological assays indicated minor toxicity and no toxicity inherent to the incorporated IG-HIP complex. SEDDS formulations F1 and F2 were administered to rats via oral gavage and resulted in a bioavailability of 0.55% and 0.44%, corresponding to a 7.7-fold and 6.2-fold increased bioavailability, respectively. Thus, incorporation of complexed insulin glargine into SEDDS formulations provides a promising approach to facilitate its oral absorption.
2.1. Materials
IG (Lantus®) was purchased from Sanofi, dialyzed at pH 4 (molecular weight cut-off 1 kDA) and subsequently lyophilized. Labrasol® ALF (caprylocaproyl polyoxyl-8-glycerides), Maisine® CC (glyceryl monolinoleate), Peceol™ (glyceryl monooleate, type 40), Plurol® oleique CC 497 (polyglyceryl-3 dioleate), Labrafac™ lipophile WL 1349 (medium-chain triglycerides), Transcutol® HP (diethylene glycol monoethyl ether) and Labrafil® M 2125 CS (lineleoyl polyoxyl-6 glycerides) were kindly provided by Gattefossé (Saint-Priest, France). Polysorbate 80 was purchased from Merck KGaA (Darmstadt, Germany). Jordapon® (sodium cocoyl isethionate) and Kolliphor® HS 15 (PEG-15 hydroxystearate) were received from BASF (Ludwigshafen, Germany). Croduret™ 50 (PEG-40 hydrogenated castor oil) was kindly provided by Croda (Nettetal, Germany). Hydrochloric acid (32%, w/w) was obtained from Carl Roth (Karlsruhe, Germany). Red blood cell concentrate was received from Tirol Kliniken GmbH (Innsbruck, Austria). Trifluoroacetic acid, sodium octadecyl sulfate and sodium taurocholate were purchased from Thermo Scientific (Vienna, Austria). All solvents (HPLC grade), oleyl alcohol and all other chemicals were obtained from Sigma-Aldrich (Vienna, Austria).
Download the full article as PDF here: Self-emulsifying drug delivery systems (SEDDS): In vivo-proof of concept for oral delivery of insulin glargine
or read it here
Victor Claus, Helen Spleis, Christoph Federer, Katrin Zöller, Richard Wibel, Flavia Laffleur, Camille Dumont, Philippe Caisse, Andreas Bernkop-Schnürch, Self-emulsifying drug delivery systems (SEDDS): In vivo-proof of concept for oral delivery of insulin glargine, International Journal of Pharmaceutics, Volume 639, 2023, 122964, ISSN 0378-5173,
https://doi.org/10.1016/j.ijpharm.2023.122964.

