Formulation of Silymarin-β Cyclodextrin-TPGS Inclusion Complex: Physicochemical Characterization, Molecular Docking, and Cell Viability Assessment against Breast Cancer Cell Lines
Abstract
Silymarin (SIL) is a poorly water-soluble flavonoid reported for different pharmacological properties. Its therapeutic applications are limited due to poor water solubility. In this study, the solubility of silymarin has been enhanced by preparing freeze-dried binary and ternary complexes using beta cyclodextrin (βCD) and d-α-tocopherol polyethylene glycol 1000 succinate (TPGS). The stoichiometry of the drug and the carrier was selected from the phase solubility study. The dissolution study was performed to assess the effect of complexation on the release pattern of SIL. The formation of inclusion complexes was confirmed by different physicochemical studies. Finally, a cell viability assay (MCF 7; breast cancer cell line) was performed to compare the activity with free SIL.
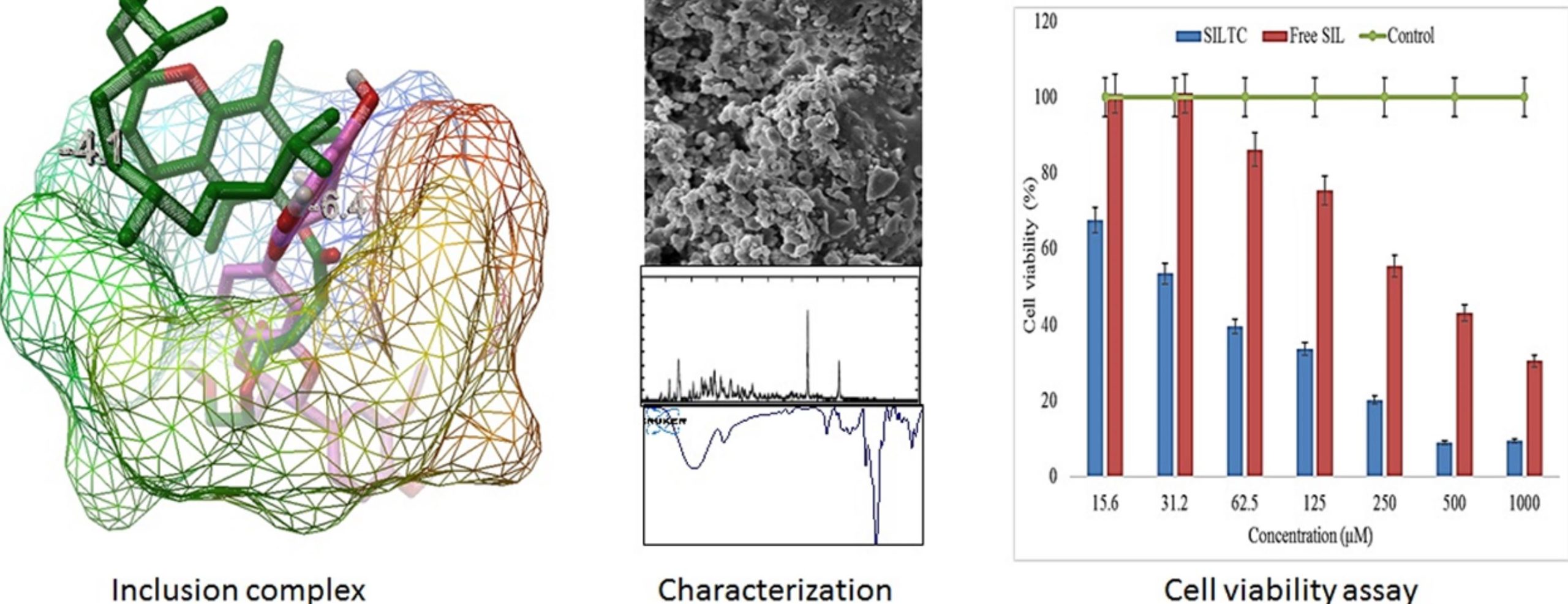
The phase solubilization results revealed the formation of a stable complex (binary) with a stability constant and complexation efficiency (CE) value of 288 mol L–1 and 0.045%. The ternary sample depicted a significantly enhanced stability constant and CE value (890 mol L–1 and 0.14%). The release study results showed a marked increase in the release pattern after addition of βCD (alone) in the binary mixture (49.4 ± 3.1%) as well as inclusion complex (66.2 ± 3.2%) compared to free SIL (32.7 ± 1.85%). Furthermore, with the addition of TPGS in SIL-βCD (ternary), the SIL release was found to be significantly enhanced from the SIL ternary mixture (79.2 ± 2.13%) in 120 min. However, fast SIL release was achieved with 99.2 ± 1.7% in 45 min for the SIL ternary complex. IR and NMR spectral analysis results revealed the formation of a stable complex with no drug–polymer interaction.
The formation of complexes was also confirmed by the molecular docking study (docking scores of 4.1 and −6.4 kcal/mol). The in vitro cell viability result showed a concentration-dependent activity. The IC50 value of the SIL ternary complex was found to be significantly lower than that of free SIL. The findings of the study concluded that the prepared SIL inclusion complex can be used as an alternative oral delivery system to enhance solubility, dissolution, and biological activity against the tested cancer cell line.
Introduction
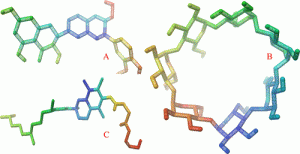
Silymarin (SIL) is a blend of four isomeric flavonoids (Figure 1A). It is derived from the milk thistle plant’s seeds and fruit. It has been reported to have low oral bioavailability, and the systemic absorption is about 23–47%. (1,2) Factors like poor water solubility, poor intestinal absorption, and instability in the gastric environment may be the reason for poor bioavailability. (3,4) The drugs with low water solubility have been associated with low biological availability due to their dissolution as a rate-limiting step for the manifestation of bioactivity. (5) To overcome these problems, very high amounts of SIL are needed to achieve biological activity. There are a plethora of research articles that report the solubility enhancement of different drugs by using formulation approaches like cyclodextrin (CD) inclusion complex, (3,6) solid dispersions, (7−9) liposomes, (10,11) microbeads, (12) and nanoparticles. (13,14)
They have shown the linkage between the solubility and bioavailability enhancement because of poor SIL solubility leading to limited drug absorption. (7,15) SIL solid dispersion was prepared by the freeze-drying method and reported significantly higher drug release than free SIL and physical mixture. It also reported many folds enhancement (215 and 589%) in the relative bioavailability as compared to the physical mixture and free SIL. (9) In another study, SIL solid dispersions were developed using the carrier polyvinylpyrrolidone-polyethylene glycol to increase the aqueous solubility. Both the carriers significantly affected the aqueous solubility and dissolution rate of SIL. They have reported ∼1150-fold enhancement in solubility with this formulation compared to plain SIL powder. (8) Another research group reported the enhanced solubility of SIL by preparing solid dispersions using d-α-tocopherol polyethylene glycol 1000 succinate (TPGS) as a carrier. They have reported marked increase in the solubility from the SIL-solid dispersion group by 23-fold compared with the SIL group. They have also reported greater permeation by 4.6-fold, and its efflux ratio decreased from 5.5 to 0.6 in the presence of TPGS. (7) The solubility enhancement of SIL was also reported by the formulation of the inclusion complex. (6) They have reported that the formulation prepared with beta cyclodextrin (βCD) showed many folds increase in drug release than free SIL. In another research study, SIL inclusion complex was prepared with hydroxypropyl β CD and randomly methylated βCD. (3) They have reported that the complexation with these two CDs shows better results as an oral delivery system. It showed a potential therapeutic efficacy in the treatment of liver fibrosis. It has been used in conventional therapy and demonstrates antioxidant, anti-inflammatory, and anticancer effects. It also can cause some cells to undergo apoptosis. It acts as an antineoplastic agent in prostate, ovarian, lung, skin, bladder, and breast cancers and other malignancies. (16,17)
CD (Figure 1B) and its derivatives are well-known solubilizers with solubility-enhancing capabilities for drugs that are not soluble in water. (18) CDs are cyclic oligosaccharides with (1–4) glucosidic linkages connecting at least 6 d(+)glucopyranose units to each other. These cyclic glucopyranose molecules form a hollow cone with an external surface that is hydrophilic and an inner cavity that is lipophilic. (19,20) Its cavity size has a diameter of 6.0–6.5 and a volume of 265 Å3, (21) and it is found suitable for different guest molecules having molecular weights between 200 and 800 g/mol. (22) In this inclusion mechanism, a lipophilic component forms a complex with the interior cavity of the CDs to enhance the solubility of the drug. Compounds can also be dissolved by CDs using simple techniques to form drug-CD complexes. (23,24) A high concentration of CD was necessary to formulate a formulation because of the high molecular weight of CD. Due to its potential toxicity and other associated side effects, the utilization of its high content may be restricted to use in delivery systems. (25) However, the low aqueous solubility of βCD is a problem for its use in drug-delivery systems. (26) The structural and functional modifications of βCD can further enhance the drug solubilization and inclusion properties due to availability of different reactive functional groups. (27,28) They enhance the aqueous solubility and wettability of poorly water-soluble drugs. (29)
According to recent reports, using a ternary system with a drug, CD, and a third auxiliary material can both lower the CD content and improve the complexation effectiveness. (25,26) There are numerous ternary substances used to prepare and evaluate inclusion complexes of poorly soluble drugs. (30−32) A novel highly water-soluble lipid-based nonionic surfactant approved as a safe excipient by the USFDA is TPGS (Figure 1C). It also has P-gp inhibitory effects and is well known to improve the solubility and bioavailability of drugs that are insoluble in water. (33,34) There is currently no literature describing the application of TPGS as a ternary substance in the formulation of inclusion complex of SIL with CD.
Using the freeze-drying technique, binary (SIL-CD) and ternary (SIL-CD-TPGS) SIL inclusion complexes were prepared in the current study design. The physical and chemical characteristics, dissolution, and cell line investigations of the prepared samples were assessed. The outcomes were compared with those of the physical mixture and free SIL. The molecular docking study evaluated the molecular formation mechanism.
Download the full article as PDF here Formulation of Silymarin-β Cyclodextrin-TPGS Inclusion Complex: Physicochemical Characterization, Molecular Docking, and Cell Viability Assessment against Breast Cancer Cell Lines
or read it here
Materials
SIL and βCD were procured from Sigma Chemicals., M.O, USA. TPGS was purchased from Wuhan Jason Biotech Co., Ltd. (Wuhan, Hubei, China). Tween 80 and ethanol were procured from Applichem, Panreac, Darmstadt, Germany, and Eurostar Scientific Ltd. Liverpool, L3 4BL, United Kingdom.
Formulation of Silymarin-β Cyclodextrin-TPGS Inclusion Complex: Physicochemical Characterization, Molecular Docking, and Cell Viability Assessment against Breast Cancer Cell Lines, Syed Sarim Imam*, Sultan Alshehri, Mohammad A. Altamimi, Wael A. Mahdi, and Wajhul Qamar, Cite this: ACS Omega 2023, Publication Date:September 11, 2023, https://doi.org/10.1021/acsomega.3c04225, Copyright © 2022 The Authors. Published by American Chemical Society. This publication is licensed under, CC-BY-NC-ND 4.0.

