Development of Clinically Optimized Sitagliptin and Dapagliflozin Complex Tablets: Pre-Formulation, Formulation, and Human Bioequivalence Studies
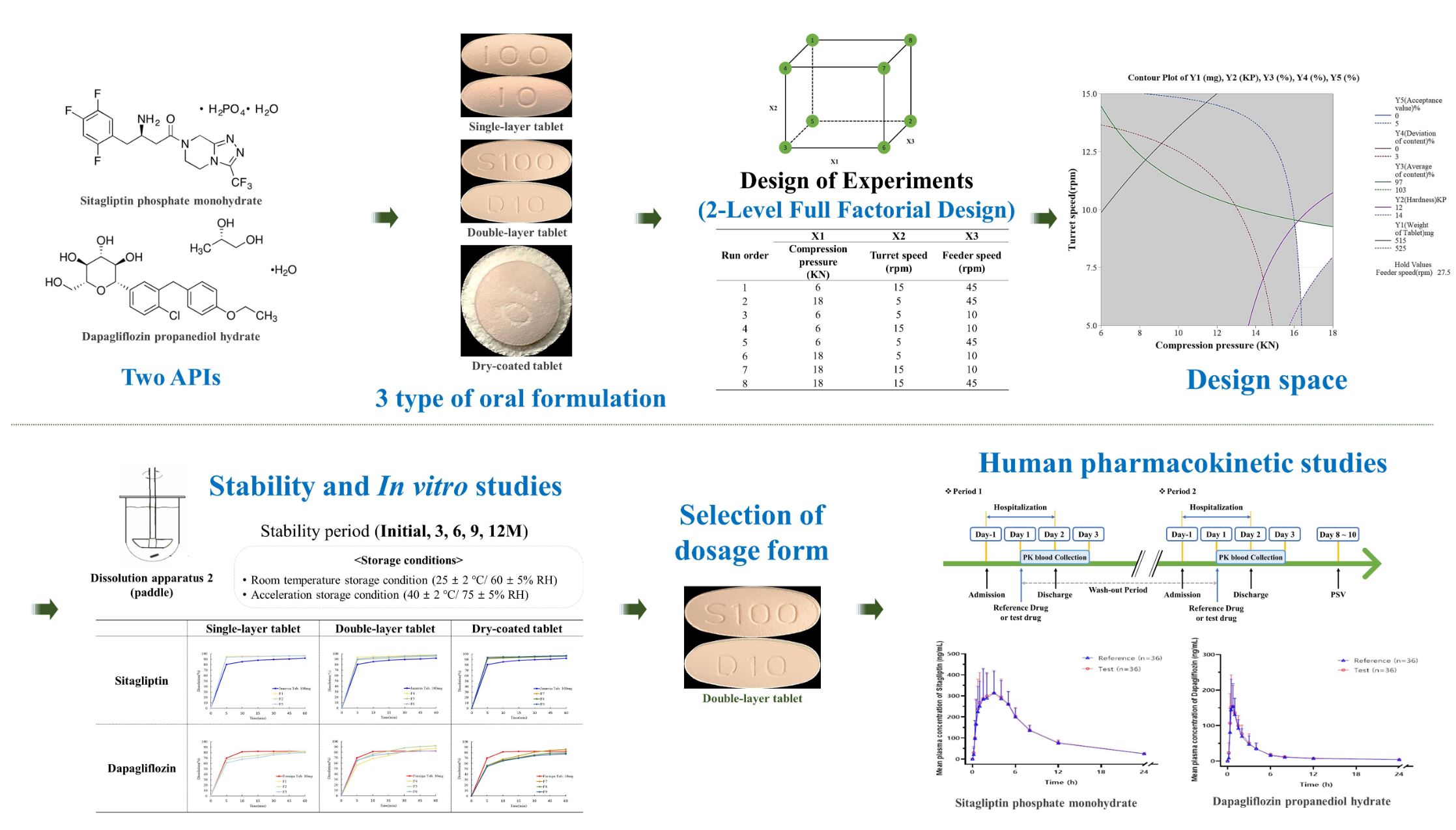
The purpose of this study is to derive an optimal drug release formulation with human clinical bioequivalence in developing a sitagliptin phosphate monohydrate-dapagliflozin propanediol hydrate fixed-dose combination (FDC) tablet as a treatment for type 2 diabetes mellitus. As a treatment for type 2 diabetes mellitus, the combined prescription of dipeptidyl peptidase-4 (DPP-4) inhibitors and sodium-glucose cotransporter-2 (SGLT-2) inhibitors is common. Therefore, this study simplified the number of individual drugs taken and improved drug compliance by developing FDC tablets containing sitagliptin phosphate monohydrate as a DPP-4 inhibitor and dapagliflozin propanediol hydrate as an SGLT-2 inhibitor. To derive the optimal dosage form, we prepared single-layer tablets, double-layer tablets, and dry-coated tablets and evaluated the drug control release ability, tableting manufacturability, quality, and stability. Single-layer tablets caused problems with stability and drug dissolution patterns. When the dissolution test was performed on the dry-coated tablets, a corning effect occurred, and the core tablet did not completely disintegrate. However, in the quality evaluation of the double-layer tablets, the hardness was 12–14 kilopond, the friability was 0.2%, and the disintegration was within 3 min.
In addition, the stability test revealed that the double-layer tablet was stable for 9 months under room temperature storage conditions and 6 months under accelerated storage conditions. In the drug release test, only the FDC double-layer tablet showed the optimal drug release pattern that satisfied each drug release rate. In addition, the FDC double-layer tablet showed a high dissolution rate of over 80% in the form of immediate-release tablets within 30 min in a pH 6.8 dissolution solution. In the human clinical trial, we co-administered a single dose of a sitagliptin phosphate monohydrate-dapagliflozin propanediol hydrate FDC double-layered tablet and the reference drug (Forxiga®, Januvia®) in healthy adult volunteers. This study showed clinically equivalent results in the stability and pharmacodynamic characteristics between the two groups.
Download the full article as PDF here Development of Clinically Optimized Sitagliptin and Dapagliflozin Complex Tablets_Pre-Formulation, Formulation, and Human Bioequivalence Studies
or read it here
Materials
Sitagliptin phosphate monohydrate was purchased from Dongbang FTL Ltd. (Hwaseong, Republic of Korea), and dapagliflozin propanediol monohydrate was purchased from Biochem Ltd. (Sejong, Republic of Korea). Dicalcium phosphate anhydrous was provided by Budenheim (Di-cafos A 150, Mainz-Bingen, Rhineland-Palatinate, Germany), and microcrystalline cellulose was supplied by JRS Pharma (Heweten 102, Holzmuhle 1, Rosenberg, Germany). Sodium starch glycolate was supplied by Roquette PTE Ltd. (GLYCOLYS, Roquette Pharma, Lestrem, France), and sodium stearyl fumarate was purchased from Anhui Sunhere Pharma (Huainan, China). Colloidal silicon dioxide was provided by Evonik Industries AG. (Aerosil 200, Essen, Germany), and crospovidone was purchased from BASF (Kollidon CL, Ludwigshafen, Germany). Magnesium stearate was purchased from Faci Asia Pacific Pte Ltd. (Merlimau PI, Jurong Island, Singapore), and silicified microcrystalline cellulose was supplied by JRS Pharma (Prosolv SMCC 90, Holzkohle 1, Rosenberg, Germany). OPADRY II was supplied by Colorcon Asia Pacific Pte Ltd. (Somerset Road, Singapore). Acetonitrile and methanol were purchased in high-performance liquid chromatography (HPLC) grade from Duksan Pharmaceutical Co. Ltd. (Ansan, Republic of Korea). pH 2.0–12.0 buffer was purchased as an extra pure grade from Duksan Pharmaceutical Co. Ltd (Ansan, Republic of Korea). Deionized water was used at 18 MΩ using a distillation device in the laboratory. All other chemicals were of analytical reagent grade and were purchased commercially.
Kang, S.-J.; Kim, J.-E. Development of Clinically Optimized Sitagliptin and Dapagliflozin Complex Tablets: Pre-Formulation, Formulation, and Human Bioequivalence Studies. Pharmaceutics 2023, 15, 1246. https://doi.org/10.3390/pharmaceutics15041246
Read more on Sodium Stearyl Fumarate as a pharmaceutical excipient here:


