Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals
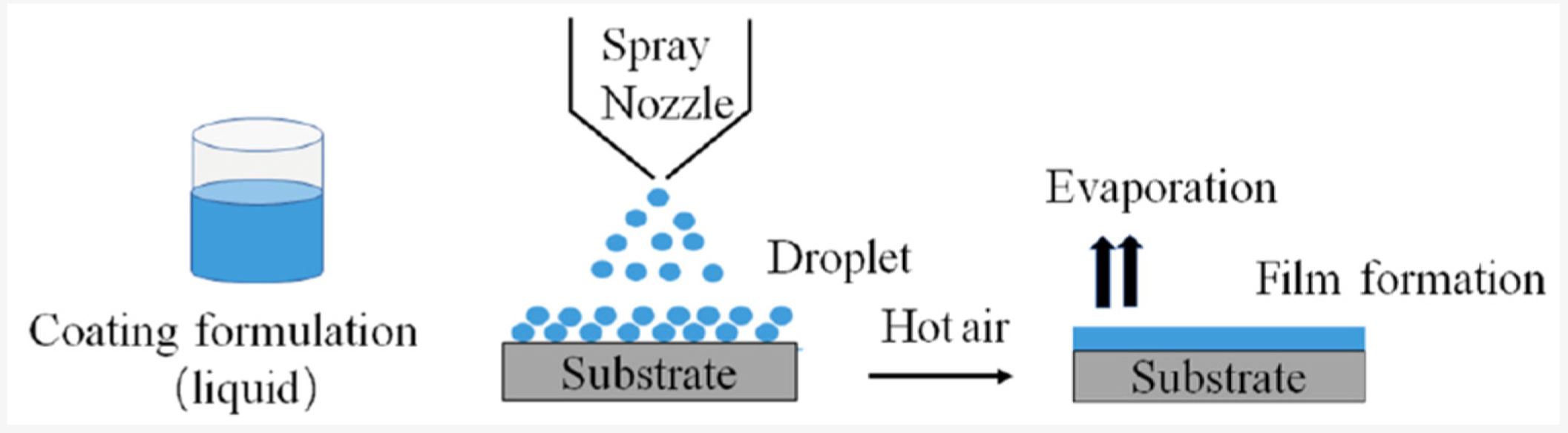
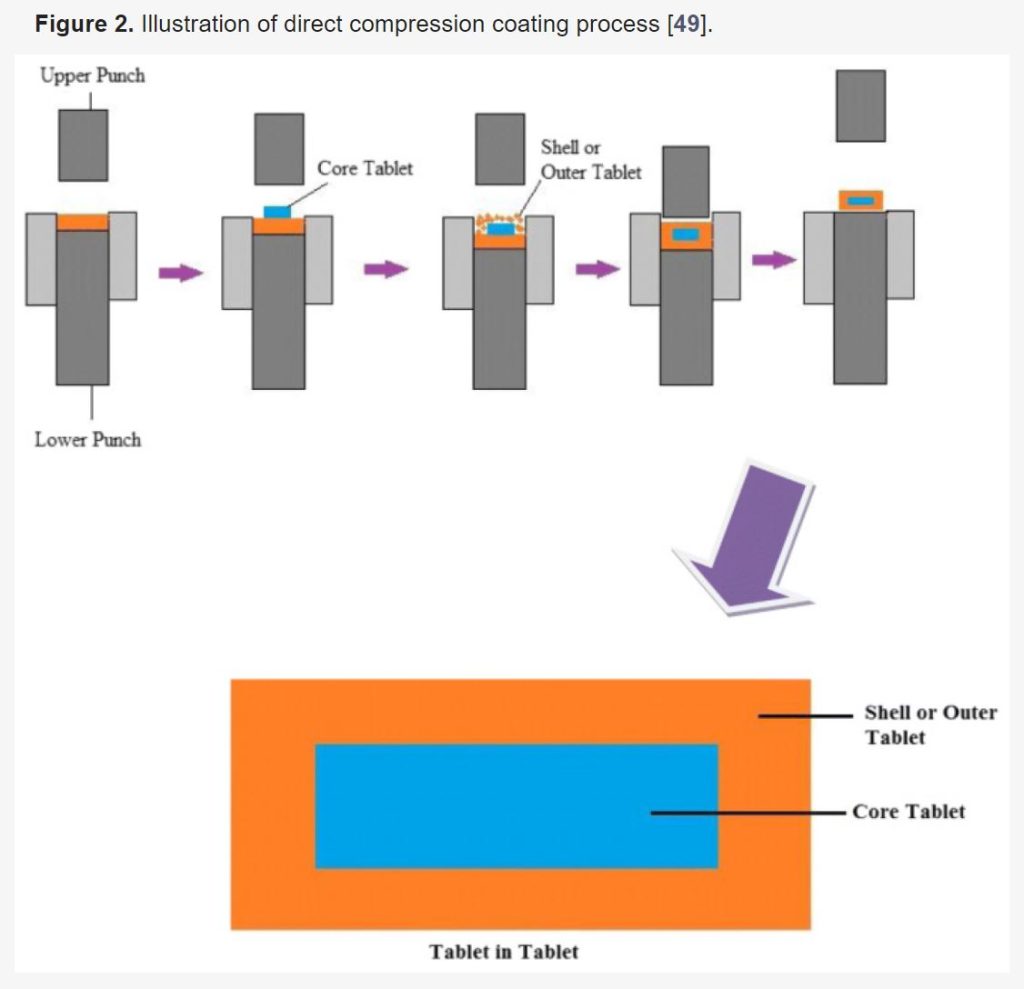
Download the full article as PDF here Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals
or read it here
Excipients mentioned in the study beside others: Eudragit L, Opadry AMB®, , Sepifilm™ LP, Eudragit EPO, Precirol® ATO 5, Compritol® 888 ATO
Ng, L.H.; Ling, J.K.U.; Hadinoto, K. Formulation Strategies to Improve the Stability and Handling of Oral Solid Dosage Forms of Highly Hygroscopic Pharmaceuticals and Nutraceuticals. Pharmaceutics 2022, 14, 2015. https://doi.org/10.3390/pharmaceutics14102015
Read more on Shellac as a pharmaceutical excipient here:


