A tabletability change classification system in supporting the tablet formulation design via the roll compaction and dry granulation process
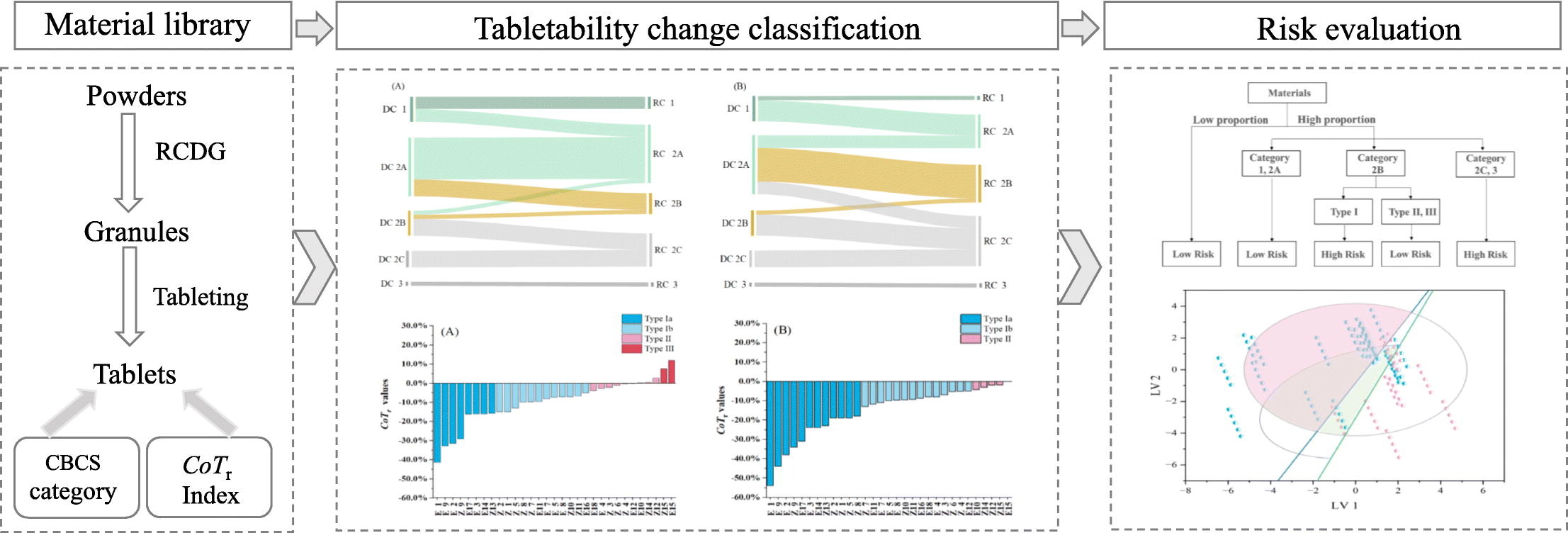
In this paper, the material library approach was used to uncover the pattern of tabletability change and related risk for tablet formulation design under the roll compaction and dry granulation (RCDG) process. 31 materials were fully characterized using 18 physical parameters and 9 compression behavior classification system (CBCS) parameters. Then, each material was dry granulated and sieved into small granules (125–250 μm) and large granules (630–850 μm), respectively. The compression behavior of granules was characterized by the CBCS descriptors, and were compared with that of ungranulated powders. The relative change of tabletability (CoTr) index was used to establish the tabletability change classification system (TCCS), and all materials were classified into three types, i.e. loss of tabletability (LoT, Type I), unchanged tabletability (Type II) and increase of tabletability (Type III).
Results showed that approximately 65% of materials presented LoT, and as the granules size increased, 84% of the materials exhibited LoT. A risk decision tree was innovatively proposed by joint application of the CBCS tabletability categories and the TCCS tabletability change types. It was found that the LoT posed little risk to the tensile strength of the final tablet, when Category 1 or 2A materials, or Category 2B materials with Type II or Type III change of tabletability were used. Formulation risk happened to Category 2C or 3 materials, or Category 2B materials with Type I change of tabletability, particularly when high proportions of these materials were involved in tablet formulation.
Highlights
- A material library was used to uncover the pattern of tabletability change in RCDG.
- The tabletability change classification system (TCCS) under RCDG was built.
- Joint use of CBCS and TCCS enabled risk decision of material’s final tabletability.
- The material tabletability design spaces for DC and RCDG were subdivided.
In addition, the risk assessment results were verified in the material property design space developed from a latent variable model in prediction of tablet tensile strength. Overall, results suggested that a combinational use of CBCS and TCCS could aid the decision making in selecting materials for tablet formulation design via RCDG.
Download the full article as PDF here A tabletability change classification system in supporting the tablet formulation design via the roll compaction and dry granulation process
or read it here
Materials
31 pharmaceutical materials, including 16 pharmaceutical excipients and 15 natural product powders (NPPs), were chosen to build a material library. In order to create a material library with representative samples, commonly used excipients with different functions such as diluents, binders and disintegrants were selected, like MCC, lactose, hydroxypropyl methylcellulose (HPMC), low-substituted hydroxypropyl cellulose (L-HPC), croscarmellose sodium (CCNa), corn starch, D-sorbitol, DCP and sodium bicarbonate (NaHCO3). 4 types of MCC and 5 types of lactose were included in the material library to expand the variation coverage of physical properties. 15 batches of NPPs prepared from 12 medicinal plant materials were provided by the Beijing Tcmages Pharmaceutical Co., Ltd. (Beijing, China). All NPPs were manufactured by pretreatment of medicinal herbs, water extraction, filtration, concentration and spray drying and could be used as raw materials for the fabrication of oral solid dosage of Chinese medicine products (Xiong et al., 2022; Yu et al., 2021; Zhang et al., 2022; Zhao et al., 2023). Compared to excipients, NPPs often possessed high hygroscopicity and poor flowability (Li et al., 2018) and could expand the physical property space of the material library. The name, lot number and supplier for each material are provided in Table S1 in Supplementary Materials.
Junhui Su, Kunfeng Zhang, Feiyu Qi, Junjie Cao, Yuhua Miao, Zhiqiang Zhang, Yanjiang Qiao, Bing Xu, A tabletability change classification system in supporting the tablet formulation design via the roll compaction and dry granulation process, International Journal of Pharmaceutics: X, Volume 6, 2023, 100204, ISSN 2590-1567, https://doi.org/10.1016/j.ijpx.2023.100204.
Read more on Disintegrants – Pharmaceutical Excipients here:


