Ring inserts as a useful strategy to prepare tip-loaded microneedles for long-acting drug delivery with application in HIV pre-exposure prophylaxis
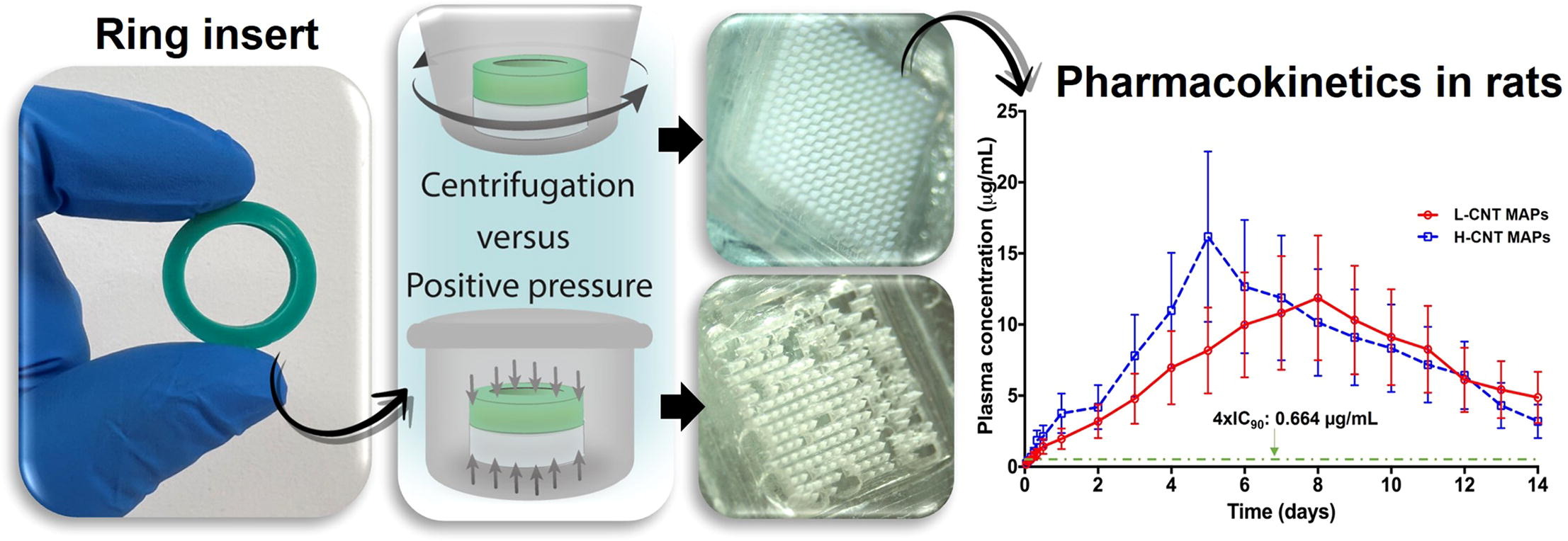
The role of microneedle array patches (MAPs) and, in particular, dissolving MAPs in transdermal drug delivery has increased exponentially over the last decade. MAPs are able to form drug depots in the viable skin from where poorly soluble drugs dissolve in a long-acting fashion, showing promise in the management of multiple diseases. The manufacture of these systems can present some challenges, including the presence of bubbles in the baseplates and consequent lack of uniformity in microneedle formation and drug content. Here, we present a simple method based on ring inserts to produce tip-loaded MAPs using the antiretroviral drug cabotegravir sodium (CAB).
Highlights
- The use of MAPs for drug delivery has gained considerable attention over the last decade.
- MAPs can deliver drugs systemically for weeks, which is desirable in HIV PrEP.
- The manufacture of MAPs is associated with several challenges, including bubble formation.
- The ring inserts developed here allowed the formation of highly uniform MAPs.
- Long-acting delivery of CAB in rats was maintained for 14 days.
The obtained MAPs presented a high uniformity in terms of microneedle formation, and a suitable insertion capability, as per the mechanical characterisation performed. An optimisation based on design of experiments revealed that centrifugation parameters had a significant impact on the skin deposition of the MAPs in excised neonatal porcine skin using Franz cells, with values ranging from 62.24 ± 47.13 µg to 174.13 ± 41.10 µg of CAB. Pharmacokinetic studies carried out in rats evidenced the capacity of the MAPs to maintain therapeutic plasma levels of CAB for 14 days, with Tmax values reached between 5 and 8 days.
Download the research paper as PDF: Ring inserts as a useful strategy to prepare tip-loaded microneedles for long-acting drug delivery with application in HIV pre-exposure prophylaxis
Materials
Cabotegravir sodium (CAB) of analytical grade was kindly provided by ViiV Healthcare (North Carolina, USA). Poly(vinylpyrrolidone) (PVP k29-32; and PVP k90) was purchased from Ashland (Kidderminster, UK). Poly(vinyl alcohol) (PVA) (9–10 kDa) was obtained from Sigma-Aldrich (Dorset, UK) and. Poly(lactic acid), PLA filament, for the 3D printing of the ring inserts master template was obtained from Ultimaker (Geldermalsen, The Netherlands). Xiameter® RTV-4250-S silicone base and curing agent for the fabrication of the ring inserts were sourced from Notcutt (Surrey, UK). Phosphate buffered saline (PBS) tablets were from Oxoid Ltd, Thermo Fischer Scientific, Massachusetts, USA. Ultrapure water was obtained from an Elga PURELAB DV 25 system (Veolia Water Systems, Dublin, Ireland) and used in all the experiments. All other reagents used in this work were of analytical grade.
Alejandro J. Paredes, Andi Dian Permana, Fabiana Volpe-Zanutto, Muhammad Nur Amir, Lalitkumar K. Vora, Ismaiel A. Tekko, Nima Akhavein, Andrew D. Weber, Eneko Larrañeta, Ryan F. Donnelly,
Ring inserts as a useful strategy to prepare tip-loaded microneedles for long-acting drug delivery with application in HIV pre-exposure prophylaxis, Materials & Design, Volume 224, 2022, 111416, ISSN 0264-1275,
https://doi.org/10.1016/j.matdes.2022.111416.

