Comparison of Different Liquid and Semisolid Vehicles Selected for Oral Administration of Pellets and Minitablets with Diazepam: In Vitro Investigation
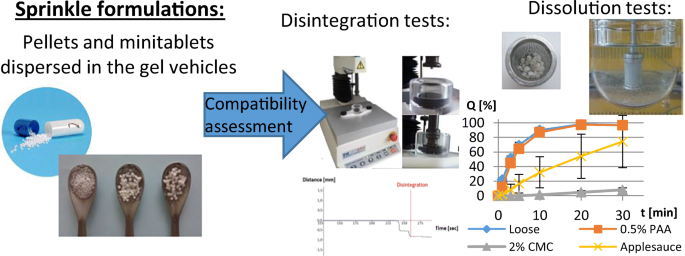
The acceptability and palatability of a dosage form are extremely important to improve patient compliance. Mixing oral solid dosage forms with food carriers is often necessary to ease swallowing and provide the taste-masking effect. The present research investigated how a liquid or semisolid carrier influences the disintegration time and drug dissolution rate of pellets and minitablets with diazepam. The disintegration of pellets and minitablets in liquid carriers (water, milk and apple juice) was determined using a texture analyser.
Dissolution tests were performed for the dosage forms dispersed in gel vehicles (2% carmellose and 0.5% carbomer gels) or applesauce. The disintegration of minitablets in water and apple juice was fast (1 min), but it slowed to 3 and 5 min in milk and gel vehicles, respectively. The pellets disintegrated in liquid carriers within 3 min. The drug dissolution rate in 0.1 M HCl depended on the gel viscosity in this medium. The preserved high viscosity of a carmellose gel inhibited the dissolution of diazepam. On the other hand, the viscosity of the carbomer gel decreased rapidly, and in effect, the dissolution rate of diazepam from the incorporated pellets or minitablets was comparable to the dissolution from loose pellets or minitablets. Access the full publication here
Conclusions
The present study showed how the type of a dispersing vehicle influenced the in vitro properties of a dosage form designed for sprinkling. An alternative method for the determination of the disintegration of pellets and minitablets in liquids, which better correlated with conditions in the mouth compared with a pharmacopoeial test, was introduced. The limitation of this method is the applicability only to low viscosity carriers because problems with the endpoint determination occurred for high viscosity gels. The standard dissolution tests performed for pellets and minitablets dispersed in semisolid vehicles demonstrated the validity of these tests during the development of sprinkle formulation.
The results showed that the carbomer gel may be an excellent vehicle for the sprinkles due to the pH-dependent viscosity because the stiff gel structure was not preserved in an acidic pH (stomach), and the viscosity of the vehicle did not influence the release of diazepam from pellets and minitablets. Furthermore, the use of a paddle apparatus may be recommended for testing these formulations rather than a basket apparatus because more reliable and reproducible results were obtained.
Access the full publication here
Materials
Diazepam was kindly donated by Polfa Tarchomin (Warsaw, Poland). The pellets were composed of microcrystalline cellulose (Vivapur PH101, JRS Pharma, Rosenberg, Germany) and lactose monohydrate (Sorbolac 400, Meggle, Wasserburg, Germany). The minitablets additionally contained croscarmellose sodium (Ac-di-sol, FMC BioPolymer, Newark, USA) as a superdisintegrant, sodium stearyl fumarate (Pruv, JRS Pharma, Rosenberg, Germany) as a lubricant and hypromellose (Pharmacoat 606, Shin-Etsu Chemical, Tokyo, Japan) as a binder. Carmellose (CMC)—high viscosity carboxymethylcellulose sodium (Sigma-Aldrich, Steinheim, Germany; viscosity of a 2.0% aqueous solution 5000 mPas) and carbomer (PAA)—and Carbopol 974P NF (Lubrizol, Brussels, Belgium; viscosity of a 0.5% aqueous solution 8000 mPas) were used to prepare gel vehicles for pellets and minitablets. Applesauce (Gerber, Nestle, Poland) was used as a soft food vehicle. Milk with 3.2% fat (Laciate, Mlekpol, Poland) and apple juice (Tymbark, Maspex, Poland) were used as liquid carriers.
Kotlowska, H., Szymanska, M. & Sznitowska, M. Comparison of Different Liquid and Semisolid Vehicles Selected for Oral Administration of Pellets and Minitablets with Diazepam: In Vitro Investigation. AAPS PharmSciTech 21, 213 (2020). https://doi.org/10.1208/s12249-020-01761-6

