Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development
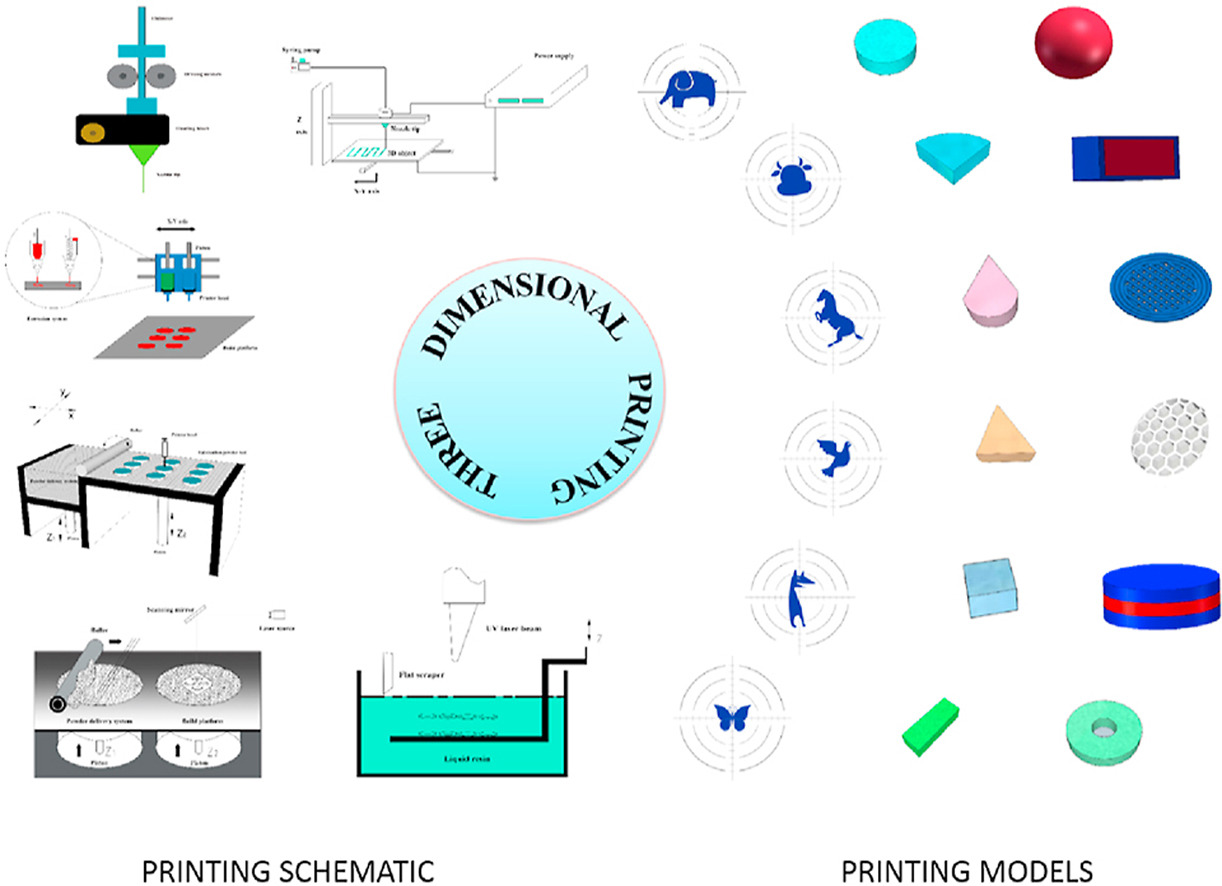
Three-dimensional printing (3D-printing) is a technology that prints the products layer-by-layer, in which materials are deposited according to the digital model designed by computer aided design (CAD) software. This technology has competitive advantages regarding product design complexity, product personalization, and on-demand manufacturing. The emergence of 3D technology provides innovative strategies and new ways to develop novel drug delivery systems. This review summarizes the application of 3D printing technologies in the pharmaceutical field, with an emphasis on the advantages of 3D printing technologies for achieving rapid drug delivery, personalized drug delivery, compound drug delivery and customized drug delivery. In addition, this article illustrates the limitations and challenges of 3D printing technologies in the field of pharmaceutical formulation development.
Read the full article (open access) here: s: Cui M, Pan H, Su Y, Fang D, Qiao S, Ding P, Pan W, Opportunities and challenges of three-dimensional printing technology in pharmaceutical formulation development, ActaPharmaceutica Sinica B, https://doi.org/10.1016/j.apsb.2021.03.015.
Excipients
In the preparation process, all types of 3D printing technologies have certain requirements on the properties of excipients due to their unique printing principles. For FDM technology, the heating and melting steps are involved in the printing process, so it is important to select a suitable drug carrier. The carrier excipient that has been reported most frequently is PVA, but its melting temperature is relatively high, which is not suitable for thermally unstable drugs, such as 4-ASA or levetiracetam. In recent years, an increasing number of researchers have attempted to combine HME technology with 3D printing technology or low-temperature 3D printing technology, using PVP, HPMC, Kollidon, talc and triethyl citrate as excipients to prepare low-temperature printed filaments to solve the problem of drug degradation and improve drug loading. For SLA and SLS technologies, the excipients are limited to photopolymers and laser sinterable materials, and these polymers are not included under the FDA’s generally recognized as safe (GRAS) list.
To date, only a few excipients have been used for printing, most of which are expensive, toxic, smelly, and need to be protected from light to avoid premature polymerization. In addition, safety manufacturing is expected for drug preparation. For DOP and SSE, one of the substantial related advantages is the feasibility of applying these technologies to various active drugs and excipients, such as epoxy resins, cheese, hydrogels, and chocolate. Even so, both technologies would relate organic solvents. In DOP technology, organic solvents are chosen as printing inks. In SSE technology, the possible addition of organic solvents is primarily adapted to prepare a soft paste. Therefore, the residual solvents in some of the final 3D printed tablets are the major limitation. According to ICH guidelines Q3C (R5), there are certain acceptance limits for the solvents, and therefore, the choice of solvents is limited, and there is a minimum tolerable residual amount for each solvent. Thus, to solve this limitation, multidisciplinary research needs to be strengthened, such as by developing new types of 3D printers.
In general, the excipients available for 3D printing technology are relatively limited in comparison with those of traditional pharmaceutical processes. Especially for special dosage forms of individual administration, selecting the printing excipients may be necessary. In addition, many of the materials applied in the printing process are nonpharmaceutical grade, and the compatibility and toxic side effects severely hinder the application of these materials in pharmaceutical formulations. Therefore, to promote the wider application of printing approaches in the pharmaceutical field, the investigation of nontoxic, biodegradable, biocompatible, and physicochemically stable excipients need to be accelerated.
Future perspectives
3D printing preparation technology and traditional preparation technology are complementary in a certain manner. Traditional preparation technology has gradually matured after a long period of practice and has unique advantages in industrialization. On the other hand, as an emerging technology, 3D printing can realize the precise shaping of a variety of materials and can overcome the issues of traditional preparation technology in many aspects. The development of printing methods in pharmaceutical formulations not only broadens the application range of 3D printing technology, but also provides new methods for pharmaceutical investigation and fosters the development of personalized drug delivery. It increases the precision and complexity of pharmaceutical formulations, and provides basic technical support for compound medicines. Although 3D printing technology still has many technical and regulatory challenges, these problems will presumably be solved in the future. In conclusion, 3D printing technology will bring new opportunities and hopes for the development of medicines, and accelerate the arrival of personalized and intelligent drug delivery.

