Dissolution Behavior of Weakly Basic Pharmaceuticals from Amorphous Dispersions Stabilized by a Poly(dimethylaminoethyl Methacrylate) Copolymer
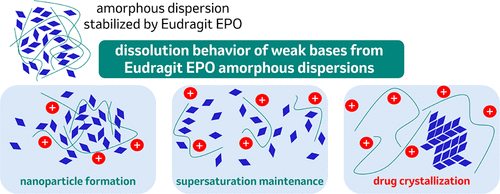
Amorphous solid dispersions (ASDs) are a well-documented formulation approach to improve the rate and extent of dissolution for hydrophobic pharmaceuticals. However, weakly basic compounds can complicate standard approaches to ASDs due to pH-dependent solubility, resulting in uncontrolled drug release in gastric conditions and unstabilized supersaturated solutions prone to precipitation at neutral pH. This work examines the release mechanisms of amorphous dispersions containing model weakly basic pharmaceuticals posaconazole and lumefantrine from a basic poly(dimethylaminoethyl methacrylate) copolymer (Eudragit EPO) and compares their dissolution behavior with ASDs stabilized by acidic and neutral polymers to understand potential benefits to release from a basic polymeric stabilizer. It was found that dissolution of Eudragit EPO ASDs resulted in supersaturation under gastric conditions, which could be sustained upon adjustment to neutral pH. However, the dissolution behavior of Eudragit EPO ASDs was sensitive to the initial pH of the gastric media. For lumefantrine, elevated initial gastric pH resulted in precipitation of amorphous nanoparticles; for posaconazole, elevated gastric pH led to crystallization of the pharmaceutical from solution. This sensitivity to gastric pH was found to originate from the impact of Eudragit EPO on gastric pH and the solubility of each pharmaceutical in the first stage of dissolution. In total, these data illustrate benefits and liabilities for the use of Eudragit EPO for ASDs containing weak pharmaceutical bases to guide the design of robust pharmaceutical formulations.

