Uncovering advances in final end-user applications, user acceptability, quality assurance, and digital technologies for 3D-printed oral drug delivery systems
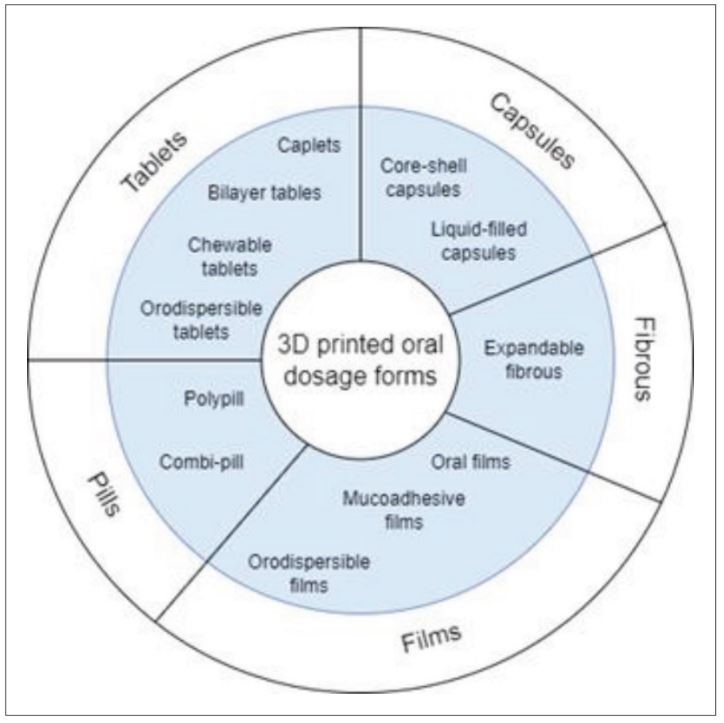
The increasing demand for innovative drugs and personalized treatment is radically changing the pharmaceutical industry, where significant efforts in research and development (R&D) are taking place. Three-dimensional (3D) printing offers interesting solutions for these demands, solving some of the limitations of current manufacturing processes. 3D-printed oral drug delivery systems can improve the delivery of pharmaceutical substances in the body, and the dynamic interaction between pharmaceutical ingredients, while providing personalized formulations, geometries, sizes, controlled release rates, and increasing time in the gastrointestinal tract. Advances in 3D printing for oral drug delivery systems have been investigated in terms of processes, materials, and effects.
However, it is important to also consider other topics, such as the specific needs of the users to enhance drugs acceptability, the quality control processes due to the absence of approved guidelines, and the digitalization of the industry to respond to future challenges of the digital era; nevertheless, there are no studies that comprise these elements. To fill this gap, the aim of this research is to identify advances in terms of final end-user applications, quality assurance, user acceptability, and digital technologies for 3D-printed oral drug delivery systems.
To accomplish this, a competitive technology intelligence (CTI) methodology was applied, where scientific literature was retrieved from the Web of Science covering the period from January 1, 1900, to May 1, 2023. For this task, a scientometric analysis was performed, and the main trends involving the previously mentioned elements were identified. In the first case, 3D-printed oral drug delivery systems are being designed for different purposes, including as anti-deterrent formulations to decrease the global problem of opioid abuse. For quality assurance, the results demonstrated the implementation of approaches like quality by design to increase the quality of the 3D-printed dosage forms. In the case of user acceptability, the interest in creating more attractive formulations was identified; for this, innovative technologies such as ColorJet 3D printing are being used. Lastly, regarding digital technologies, the importance of cyberattacks while sending the 3D-printed dosage form file to the 3D printer is highlighted; for this, cybersecurity systems are being studied. The outcomes of this study can add value to researchers, organizations, and investment firms interested in the R&D of novel and personalized treatments, and the areas of 3D printing, pharmaceutical, medical, and health.
Table 1. Excipients utilized in various 3D printing techniques
| Process | Technique | Material (excipients) | Reference |
|---|---|---|---|
| Binder jetting | Binder jetting | – Polyvinylpyrrolidone (PVP) – Kollidon® SR – Hydroxypropyl methylcellulose (HPMC) – Polyvinylpyrroldine-vinyl (PVPV) – Hydroxypropyl cellulose (HPC) – Ethylcellulose (EC) – Mycrocrystalline cellulose (MCC) – Lactose monohydrate – Methyl cellulose (MC) | [13] |
| Material extrusion | Fused deposition modeling | – Polylactic acid (PLA) – Polyvinyl alcohol (PVA) – Soluplus – Ethylcellulose (EC) – Eudragit® – Hydroxymethyl cellulose (HMPC) – Hydroxypropyl cellulose (HPC) – Polycaprolactone (PCL) – Polyethylene glycol (PEG) – Hydroxypropyl methylcellulose (HPMC) – Polyethylene oxides (PEOs) – Polyvinylpyrrolidone (PVP) | [14] |
| Pressure-assisted microsyringe | – Hydroxymethyl cellulose (HMPC) – Mycrocrystalline cellulose (MCC) – Polyvinylpyrrolidone (PVP) – Lactose – Hydroxypropyl cellulose (HPC) – Hydroxypropyl methylcellulose (HPMC) – Polyvinyl alcohol (PVA) – Kollicoat® – Ethylcellulose (EC) – Hydroxypropyl methylcellulose (HPMC) | [15] [16] [17] |
|
| Material jetting or inkjet printing | Drop-on-demand | – Polycaprolactone (PCL) – Polyvinylpyrrolidone (PVP) – Poly (N-isopropylacrylamind-co-acrylamide) (PNIPAM) – Hydroxypropyl methylcellulose (HPMC) – Hydroxypropyl cellulose (HPC) | [18] [19] |
| Continuous inkjet printing | – Hydroxypropyl methylcellulose (HPMC) – Hydroxypropyl cellulose (HPC) – Polyethylene glycol (PEG) | [20] | |
| Powder bed fusion | Selective laser sintering | – Polycaprolactone (PCL) – Eudragit® – Kollicoat® IR – Hydroxymethyl cellulose (HMPC) – Poly-I-lactic acid (PLLA) – Kollidon® – Mycrocrystalline cellulose (MCC) – Ethylcellulose (EC) – Polyethylene oxide (PEO) – Methylcellulose (MC) | [21] |
| Vat photopolymerization | Stereolithography | – Polycaprolactone (PCL) – Polyethylene glycol diacrylate (PEGDA) – Polyethylene glycol dimethacrylate (PEGDMA) – Poly (2-hydroxyethyl methacrylate) (pHEMA) – Polypropylene fumarate (PPF) – Diphenyl (2,4,6-trimethylbenzoyl) (TPO) – Polyethylene glycol (PEG) – Triol | [22] [23] [24] |
| Digital light processing | – Polyethylene glycol diacrylate (PEGDA) – Polyethylene glycol (PEG) – Acrylated hyperbranched polyester (AHBPE) – Polyethylene glycol dimethacrylate (PEGDMA) – Polycaprolactone (PCL) – Polypropylene fumarate (PPF) – Diphenyl (2,4,6-trimethylbenzoyl) phosphine oxide (TPO) | [22] [25] [26] [24] |
Download the full research as PDF here: Uncovering advances in final end-user applications, user acceptability, quality assurance, and digital technologies for 3D-printed oral drug delivery systems
or read it here
Veronica L. Rios-Mata ,Marisela Rodriguez-Salvador ,Jia An ,Chee Kai Chua ,Pedro F. Castillo-Valdez. Uncovering advances in final end-user applications, user acceptability, quality assurance, and digital technologies for 3D-printed oral drug delivery systems. IJB null, 0(0), 1119.
https://doi.org/10.36922/ijb.1119

