Orally Administered Amphotericin B Nanoformulations: Physical Properties of Nanoparticle Carriers on Bioavailability and Clinical Relevance
Amphotericin B is an effective polyene antifungal considered as a “gold standard” in the management of fungal infections. Currently, it is administered mainly by IV due to poor aqueous solubility, which precludes its delivery orally. Paradoxically, IV administration is akin to side effects that have not been fully eliminated even with more recent IV formulations. Thus, the need for alternative formulations/route of administration for amphotericin B remains crucial. The oral route offers the possibility of delivering amphotericin B systemically and with diminished side effects; however, enterocyte permeation remains a constraint. Cellular phagocytosis of submicron particles can be used to courier encapsulated drugs. In this regard, nanoparticulate delivery systems have received much attention in the past decade. This review examines the trajectory of orally delivered amphotericin B and discusses key physical factors of nanoformulations that impact bioavailability. The review also explores obstacles that remain and gives a window into the possibility of realizing an oral nanoformulation of amphotericin B in the near future.
Table 1. Summary of in vitro and in vivo studies on different types of oral AmpB nanoformulations.
| Drug Delivery System | Method of Preparation, Major Components and Key Features | Outcome | Ref. |
|---|---|---|---|
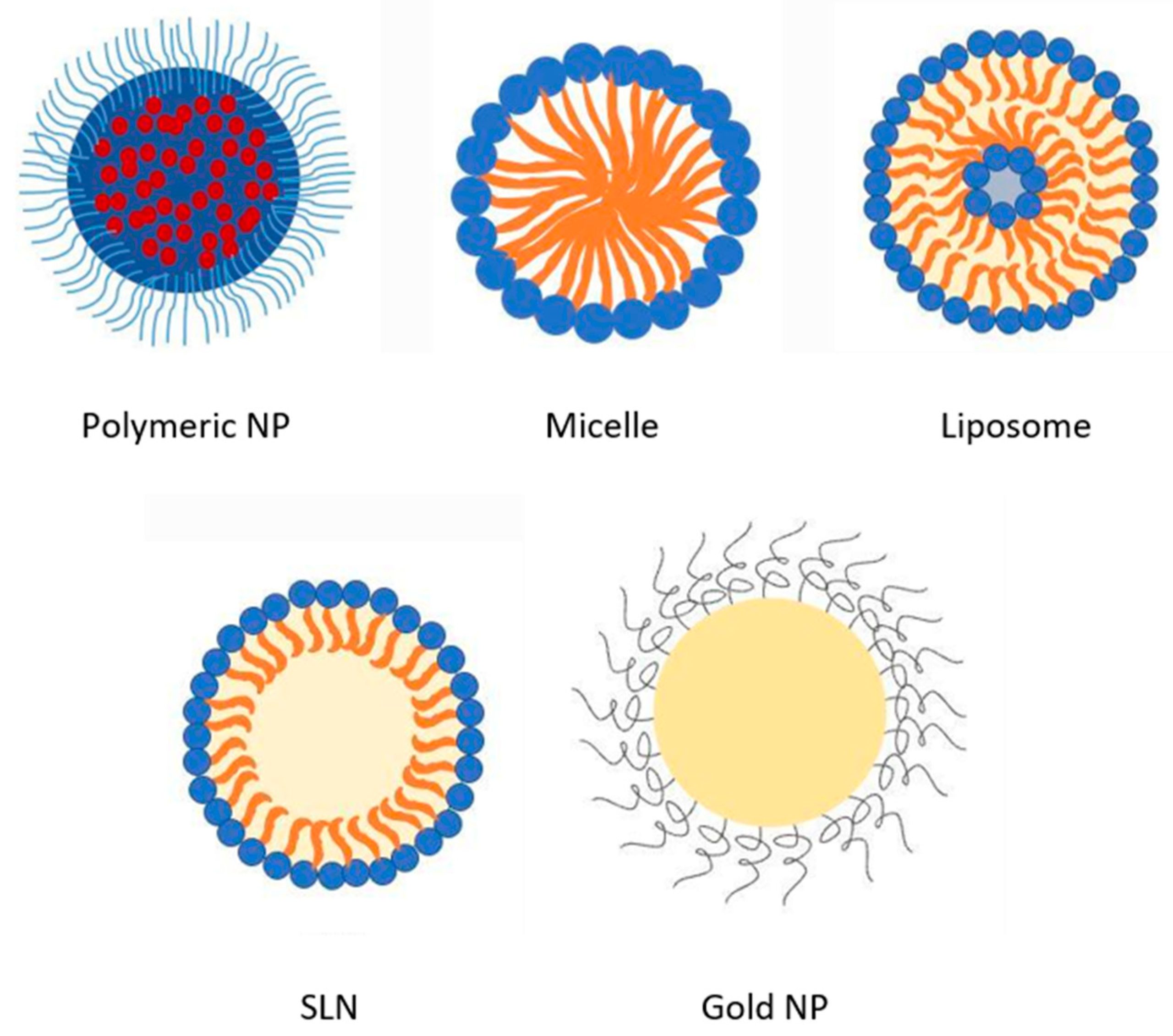
| Nanoparticles Nanosuspensions | Nanoprecipitation: PLGA, poloxamer 188 and 388. Size: 86–153 nm; ZP −31.0 mV. Solvent-antisolvent precipitation: PVA, DMSO. Size: 118–400 nm; ZP −20 mV. | AMP-B-loaded NP showed 2-fold and AmpB nanosuspensions showed 4-fold enhancement in antifungal activity compared to AmBisome® in a mouse model | [35] |
| Mannose anchored thiomer nanocarriers | Covalent linkage of thioglycolic-chitosan followed by mannose addition: Mannose, chitosan. Size: 430–482 nm | 6-fold increase in oral BA and 3-fold increase in half-life with ignificantly less toxicity than the AmpB control. | [36] |
| Liposomes | Modified injection method: Egg yolk phosphatidylcholine, cholesterol, ceramides. Size: 200 nm, EE > 75% | In an in vitro stomach–duodenum model, ceramides offered better membrane stability to ceramics anchored liposomes. Moreover, ceramides inhibited the detergent effect of bile salts on liposome membranes. | [37] |
| Cubosomes | High-pressure homogenization: Glyceryl monoolein, poloxamer 407. Size: 192 nm, EE > 94% | AmpB-loaded glyceryl monoolein cubosomes showed enhanced therapeutic efficacy than Fungizone®. A two-day treatment of 10 mg/kg dose was sufficient to attain therapeutic concentrations at the renal tissues for fungal treatment. | [38] |
| Solid lipid nanoparticles (SLN) | Nanoprecipitation followed by probe sonication: Glyceride dilaurate, phosphatidylcholine, PEG-660–12 hydroxystearate. Size: 200 nm, EE > 95% | In vivo pharmacokinetics evaluation on Wistar rats showed faster onset of action and prolonged half-life than pure drug solution. | [30] |
| Nanostructured lipid carriers (NLC) | Homogenization ultrasonication: Chitosan, beeswax, coconut oil. Size: 394 nm, EE > 86% | NLC formulation showed comparable antifungal (in vitro) efficacy than AmpB and is twice less toxic to RBCs. | [39] |
| Stealth nanoparticles | Emulsification diffusion: PLGA-PEG copolymers, PVA, vitamin E, pluronic F68. Size: <1000 nm, EE > 56% | PEG concentration plays a significant role in size, EE, and drug release. 15% PEG demonstrated a controlled drug release of 54% up to 24 h. | [40] |
| Nanocapsules | Nanoemulsion production by emulsion solvent evaporation followed by chitosan deposition. Mannose sugar, chitosan, soya lecithin, polysorbate 80. Size:198 nm; ZP +31 mV, EE 96%. | Increased macrophage selectivity by interacting with overexpressed mannose receptors, resulting in 90% reduction in spleen parasite load and decreased nephrotoxicity. | [41] |
| Lipopolymerosome | Single-step nanoprecipitation: Glycol–chitosan, stearic acid, soya lecithin, cholesterol. Size: 243 nm; ZP +27 mV. | In vitro and in vivo evaluation demonstrated improved plasma drug stability and reduced toxicity compared to AmBisome® and Fungizone®. Enhanced anti-leishmanial activities and the glycol–chitosan copolymer was vital in improving the drug stability. | [42] |
| Polymer–lipid hybrid nanoparticles (PLN) | Desolvation method: Gelatin, lecithin, acetone, DMSO. Size: 253 nm, EE > 50.0% | Drug release followed Huguchi kinetics. Moreover, a 6-fold increase in intestinal permeability on Caco-2 cell lines and a 5-fold enhancement in oral BA was found compared to free AmpB. | [34] |
| Nanocochleates | Film hydration: Phosphatidylserine, lecithin, cholesterol, vitamin E. ZP −9 to −16 mV, EE > 50.0%. | Enhanced gastric stability and slow release in the GI medium. A confocal microscopy study demonstrated the integration of phosphatidylserine to the Caco-2 intestinal cell layers and slow drug release. | [43] |
| Self-emulsifying drug delivery systems (SEDDS) | Solvent evaporation: Glyceryl mono-oleate, PEG, phospholipids. Size: 200–400 nm, enhanced solubility stability in SGF or SIF | SEDDS significantly decreased fungal CFU in Sprague–Dawley rats infected with A. fumigatus and Candida albicans without causing renal toxicities. | [44] |
| Polymeric micelles | Solvent-diffusion and microfluidics technique: Copolymer Soluplus® (Polyvinyl caprolactam–PVA–PEG), VitE-TPGS Size: 80 nm, EE 95.0% | Enhanced cell uptake (6-fold) and permeability (2-fold) in Caco-2 cells in vitro while being less toxic than free AmpB. | [45] |
| Nanofibers | Electrospinning technique. PLGA, chloroform, 2,2,2-trifluoroethanol. Size: 582 nm, controlled delivery of AmpB for 8 days. | For vulvovaginal candidiasis, the vaginal fungal load in a murine model was eliminated after three days of local treatment. | [46] |
Download the full review as PDF here Orally Administered Amphotericin B Nanoformulations: Physical Properties of Nanoparticle Carriers on Bioavailability and Clinical Relevance
or read it here
Fairuz, S.; Nair, R.S.; Billa, N. Orally Administered Amphotericin B Nanoformulations: Physical Properties of Nanoparticle Carriers on Bioavailability and Clinical Relevance. Pharmaceutics 2022, 14, 1823. https://doi.org/10.3390/pharmaceutics14091823

