Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery
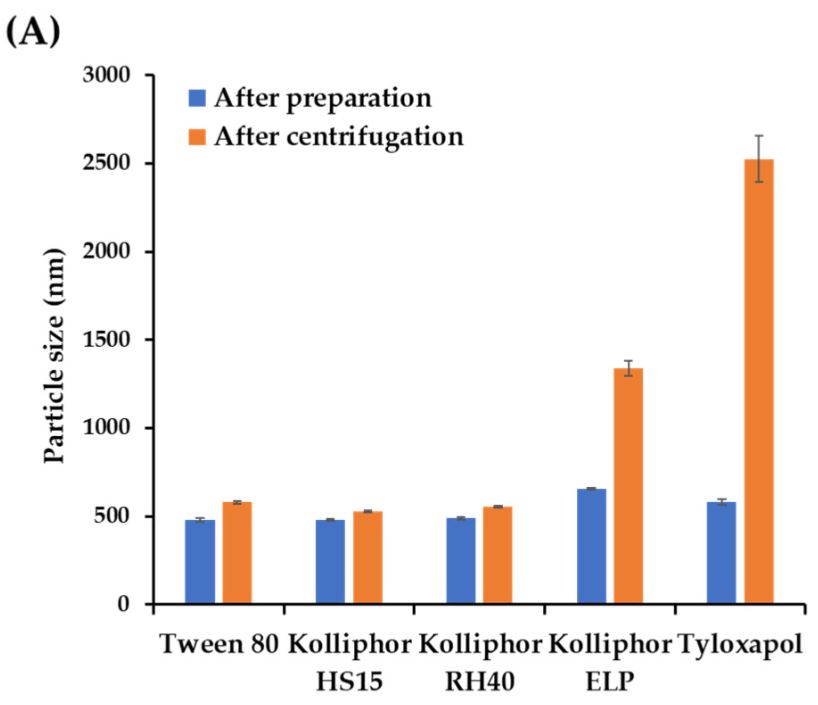
A high-payload ascorbyl palmitate (AP) nanosuspension (NS) was designed to improve skin delivery following topical application. The AP-loaded NS systems were prepared using the bead-milling technique, and softly thickened into NS-loaded gel (NS-G) using hydrophilic polymers. The optimized NS-G system consisted of up to 75 mg/mL of AP, 0.5% w/v of polyoxyl-40 hydrogenated castor oil (Kolliphor® RH40) as the suspending agent, and 1.0% w/v of sodium carboxymethyl cellulose (Na.CMC 700 K) as the thickening agent, in citrate buffer (pH 4.5). The NS-G system was embodied as follows: long and flaky nanocrystals, 493.2 nm in size, −48.7 mV in zeta potential, and 2.3 cP of viscosity with a shear rate of 100 s−1.
Both NS and NS-G provided rapid dissolution of the poorly water-soluble antioxidant, which was comparable to that of the microemulsion gel (ME-G) containing AP in solubilized form. In an ex vivo skin absorption study using the Franz diffusion cell mounted on porcine skin, NS-G exhibited faster absorption in skin, providing approximately 4, 3, and 1.4 times larger accumulation than that of ME-G at 3, 6, and 12 h, respectively. Therefore, the high-payload NS makes it a promising platform for skin delivery of the lipid derivative of ascorbic acid.
Download the full article as PDF here Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery
or read it here
Materials
AP powder (purity over 98 w/w%), two grades of sodium carboxymethyl cellulose (Na.CMC) with an average molecular weight of ~90,000 and ~7,000,000 (named 90 K and 700 K), respectively, tyloxapol, Tween 80, xanthan gum, citric acid, and trifluoroacetic acid (TFA) were purchased from Sigma-Aldrich (St. Louis, MO, USA). Sodium lauryl sulfate (SLS) was obtained from Junsei Honsha Co., Ltd. (Tokyo, Japan). Polyvinylpyrrolidone K17 (Plasdone K 17) (PVP K17), macrogol (15) hydroxystearate (Kolliphor® HS15), macrogolglycerol ricinoleate (Kolliphor® ELP), macrogolglycerol hydroxystearate (Kolliphor® RH40), polyoxyethylene (160) polyoxypropylene (30) glycol (Poloxamer® 188 (Kollliphor P 188)), and carboxypolymethylene (Carbopol® 934NF) were acquired from CTC Bio Inc (Gyeonggi, Republic of Korea). Hydroxyethyl cellulose (Natrosol™ 250, HEC), HPC E50 LV (Methocel™ E50), and HPMC (Methocel™ F4M) were kindly provided by Whawon Pharm. Co., Ltd. (Gyeonggi, Republic of Korea). Polyethylene glycol 4000 (PEG4000), capric triglyceride (Miglyol® 812), caprylocaproyl polyoxyl (8) glycerides (Labrasol®), and polyglyceryl (3) dioleate (Plurol® oleique) were kindly provided by Masung & Co., Ltd. (Seoul, Republic of Korea). Methanol and acetonitrile (ACN) were obtained from J.T. Baker (Phillipsburg, NJ, USA) and used without additional refinement.
Park, J.-S.; Choi, J.-H.; Joung, M.-Y.; Yang, I.-G.; Choi, Y.-S.; Kang, M.-J.; Ho, M.-J. Design of High-Payload Ascorbyl Palmitate Nanosuspensions for Enhanced Skin Delivery. Pharmaceutics 2024, 16, 171. https://doi.org/10.3390/pharmaceutics16020171
Read more on Orally Disintegrating Tablets (ODTs) here:


